Nes, GINSBURG, 1933
|
publication ID |
https://doi.org/ 10.1111/zoj.12394 |
|
publication LSID |
lsid:zoobank.org:pub:E952647E-1571-4A14-8BD4-54D1746760D0 |
|
persistent identifier |
https://treatment.plazi.org/id/03A0C25D-BB6A-FF86-B7BC-FF02FC5BEC47 |
|
treatment provided by |
Marcus |
|
scientific name |
Nes |
| status |
|
NES GINSBURG, 1933 View in CoL View at ENA
TYPE SPECIES: NES LONGUS (NICHOLS, 1914) (GINSBURG, 1933: 25, DESCRIBED AS GOBIOSOMA LONGUM NICHOLS, 1914, BY ORIGINAL DESIGNATION; PROPOSED AS A SUBGENUS OF GOBIOSOMA )
Diagnosis
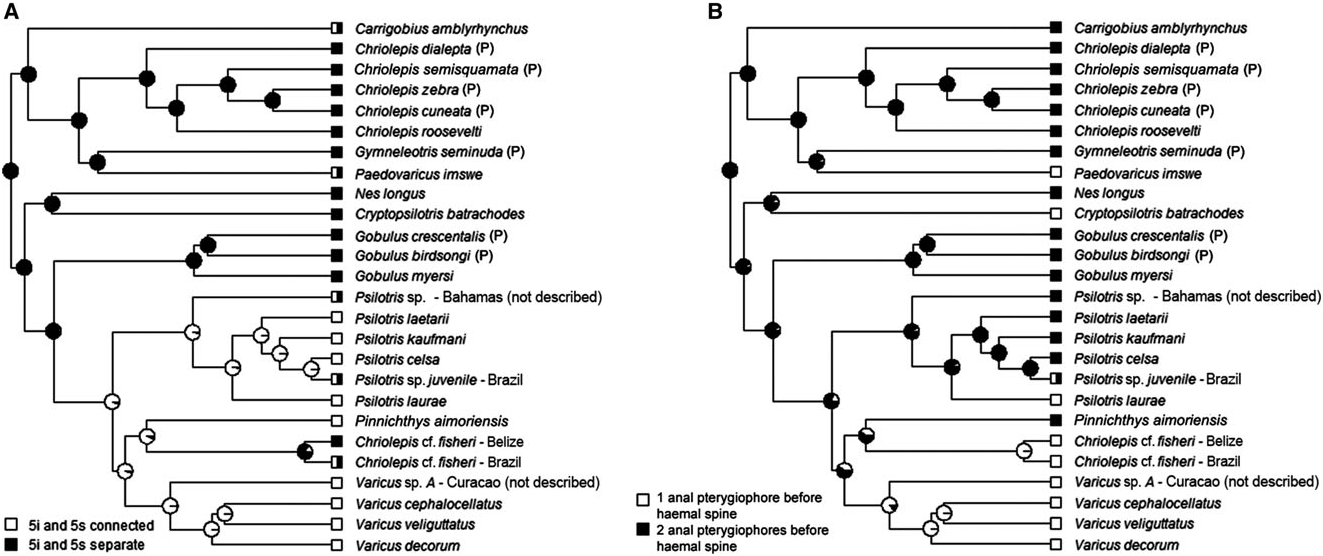
Possesses all taxonomic characters present in most members of Gobiosomatini and Gobiosoma group (first dorsal-fin spines VII, pterygiophore insertion pattern of 3 – 221110, 27 vertebrae – 11 precaudal and 16 caudal, hypurals 1 and 2 fused to some extent with hypurals 3 and 4 and the terminal vertebral element, one epural); pelvic fins united completely to form round to oval disc, with well-developed anterior frenum uniting pelvic spines; pelvic-fin rays 1 – 5 branched; pelvic-fin rays extending posteriorly to point halfway to anus or slightly further, never reaching anus; side of body without scales (modified basicaudal scales absent); two anal-fin pterygiophores inserted before first haemal spine; papillae rows 5i and 5s separate; cephalic lateralis canals and pores absent; second dorsal-fin rays I,10 – 13; anal-fin rays I,10 – 12; body variously mottled and with distinct series of horizontally paired dark spots along lateral midline. The one known species occurs in the western Atlantic Ocean.
Remarks
The genus Nes contains one species, Nes longus . The ecology and behaviour of this species has been extensively studied because of its interesting association with Alpheus snapping shrimp ( Randall, Lobel & Kennedy, 2005; Kramer, Van Tassell & Patzner, 2009). It is the only species in the Nes subgroup of the Gobiosomatini to have a fully developed pelvic disc. The phylogenetic position of Nes based on our molecular tree is unclear. A sister relationship between Nes and Psilotris batrachoides (now assigned to Cryptopsilotris gen. nov.) is shown in Fig. 5 View Figure 5 , but support is weak (Bayesian posterior probability = 0.62).
PAEDOVARICUS VAN TASSELL, TORNABENE & GILMORE View in CoL GEN. NOV. TYPE SPECIES: PAEDOVARICUS IMSWE View in CoL ( GREENFIELD, 1981: 269, DESCRIBED AS VARICUS IMSWE View in CoL )
Diagnosis
Possesses all taxonomic characters present in most members of Gobiosomatini and Gobiosoma group (first dorsal-fin spines VII, pterygiophore insertion pattern of 3 – 221110, 27 vertebrae – 11 precaudal and 16 caudal, hypurals 1 and 2 fused to some extent with hypurals 3 and 4 and the terminal vertebral element, one epural); pelvic fins well separated, lacking both anterior frenum and well-developed membrane connecting innermost rays; pelvic-fin rays 1 – 5 unbranched, without flattened or fleshy tips; pelvicfin rays very long, fourth ray extending posteriorly to origin of last anal-fin ray or beyond; body with scales (modified basicaudal scales present); one analfin pterygiophore inserted before first haemal spine; cephalic lateralis canals and pores absent; second dorsal-fin rays I,7; anal-fin rays I,7; body, head, and fins with light yellowish orange hue, diffuse dark vertical bars alongside of body, and very dark wide vertical band over posterior end of caudal peduncle and on base of caudal rays. The one known species occurs in the western Atlantic Ocean.
Remarks on the genus Paedovaricus
We erect the new genus Paedovaricus for Varicus imswe Greenfield, 1981 ; which was described based on two specimens from 21 to 25 m depth off Carrie- Bow Cay, Belize. This species is recovered outside of the well-supported clade containing all other species now considered to belong to Varicus . It was originally placed in Varicus based primarily on the presence of body scales, the absence of cephalic lateralis pores, and the presence of separate pelvic-fin rays with unbranched tips. The absence of cephalic lateralis pores is apparently a plesiomorphic condition within the Nes subgroup, and we now know that Varicus species may have pelvic-fin rays that are branched, unbranched, flattened at the tips, or with fleshy pads, and that there may be an ontogenetic component to these features ( Gilmore, 1979; Hastings & Bortone, 1981). Paedovaricus imswe also shares with Varicus the presence of a single anal-fin pterygiophore inserted anterior to the first haemal spine, a character that overall shows some significant phylogenetic signal ( Fig. 6B View Figure 6 ; Table 1), but has apparently evolved independently in Varicus , Paedovaricus , and Cryptopsilotris . The distinguishing features separating Paedovaricus from Varicus are the length of the pelvic-fin rays (extending to base of last anal-fin ray in Paedovaricus ; never extending past base of fourth ray in Varicus ), lower pectoral-ray counts (15 or fewer in Paedovaricus ; 16 or more in Varicus ) and second dorsal-fin rays (I, 7 in Paedovaricus ; I,8 or more in Varicus ). The depth range for Paedovaricus is 15 – 32 m, whereas all other Varicus species occur from deeper waters (typically> 60 m, with most species found> 150 m); however, Paedovaricus may be more common in deeper water but rarely collected because of its small size. The largest known specimen is 13.5 mm SL, and individuals are apparently sexually mature at 8.0 mm SL ( Williams & Gilbert, 1983). In comparison, Varicus reach much larger sizes, which may be associated with different selective pressures associated with living at greater depths. For example, the largest and deepest known species of Varicus , Varicus adamsi sp. nov., was collected at a depth of 435 m and was 61 mm SL.
According to our molecular phylogenetic analysis ( Fig. 5 View Figure 5 ) the closest relative to Paedovaricus is the eastern Pacific species Gymneleotris seminuda (G unther €, 1864); however, the statistical support for this sister relationship is low (0.69 posterior probability), and these two species differ in most of the phylogenetically informative morphological characters examined here. Gymneleotris lacks the modified basicaudal scales that are present in Paedovaricus . All pelvic rays are unbranched in Paedovaricus , whereas all rays (including the well-developed fifth pelvic ray) are branched in Gymneleotris . Head pores are present in Gymneleotris and lacking in Paedovaricus . Gymneleotris has two anal-fin pterygiophores inserted before the haemal spine, whereas Paedovaricus has one. Lastly, the two species have very different coloration, with Paedovaricus being cryptically mottled with an overall yellowish orange tone ( Fig. S5D View Figure 5 ), and with Gymneleotris having prominent alternating black and white vertical bars over the head and body ( Figs S6B and S View Figure 6 7F View Figure 7 ). Thus, we maintain the two as separate monotypic genera.
Etymology
The genus name Paedovaricus is formed from the root ‘paed-’ (the English spelling of the Greek root ‘ped-’, meaning ‘child’) and Varicus . The name is in reference to the small size of the type species Paedovaricus imswe and its general similarity to the genus Varicus .
PINNICHTHYS VAN TASSELL, TORNABENE & GILMORE GEN. NOV. TYPE SPECIES: PINNICHTHYS AIMORIENSIS VAN TASSELL, TORNABENE & GILMORE SP. NOV
Diagnosis
Possesses all taxonomic characters present in most members of Gobiosomatini and Gobiosoma group (first dorsal-fin spines VII, pterygiophore insertion pattern of 3 – 221110, 27 vertebrae – 11 precaudal and 16 caudal, hypurals 1 and 2 fused to some extent with hypurals 3 and 4 and the terminal vertebral element, one epural); pelvic fins well separated, lacking both anterior frenum and well-developed membrane connecting innermost rays; pelvic-fin rays 1 – 4 branched, sometimes with flattened tips, fifth pelvic-fin ray unbranched; pelvic-fin rays extending posteriorly to anus or beyond, rarely reaching origin of first anal-fin ray; body with scales (modified basicaudal scales present); two anal-fin pterygiophore inserted before haemal spine; papillae rows 5i and 5s connected, or nearly so, separated by the absence of a single papilla in one species; cephalic lateralis canals and pores absent; second dorsal-fin rays I,10 – 11; anal-fin rays I,10 – 11; first dorsal-fin spine elongate in two species; coloration in life known only for two species ( Pinnichthys aimoriensis gen. et sp. nov. and Pinnichthys saurimimica gen. et sp. nov.), and consists primarily of four or five dark-brown or brownish yellow botches along lateral midline, with four or five pairs of short narrow saddles along dorsal midline; in preservation, side of body pale with series of evenly-spaced dark spots, saddles, or broken saddles. Four species occur in the western Atlantic Ocean and one species occurs in the eastern Pacific Ocean.
Remarks on the genus Pinnichthys
The genus Pinnichthys is erected based on the type species Pinnichthys aimoriensis gen. et sp. nov., a new species collected from a depth of 70 m near the edge of the continental slope off Espirito Santo, Brazil. Our phylogeny shows Pinnichthys aimoriensis gen. et sp. nov. nested within a clade of western Atlantic species that also includes a monophyletic Varicus clade, a monophyletic Psilotris clade, and specimens tentatively identified as Chriolepis fisheri ( Fig. 5 View Figure 5 ). An alternative phylogenetic classification to the one proposed here would be to include Pinnichthys aimoriensis gen. et sp. nov. and Chriolepis cf. fisheri as part of Varicus . Pinnichthys aimoriensis gen. et sp. nov. (and the other four species assigned here to Pinnichthys ) differs from Varicus in several ways, however: (1) the body of Pinnichthys has more scales than Varicus (lateral scale rows 0 – 27 vs. 30 – 53); (2) the counts in the anal fin are higher in Pinnichthys than in Varicus (I,10 – 11 vs. I,7 – 9); and (3) Pinnichthys has two anal-fin pterygiophores inserted anterior to the haemal spine, whereas Varicus has one.
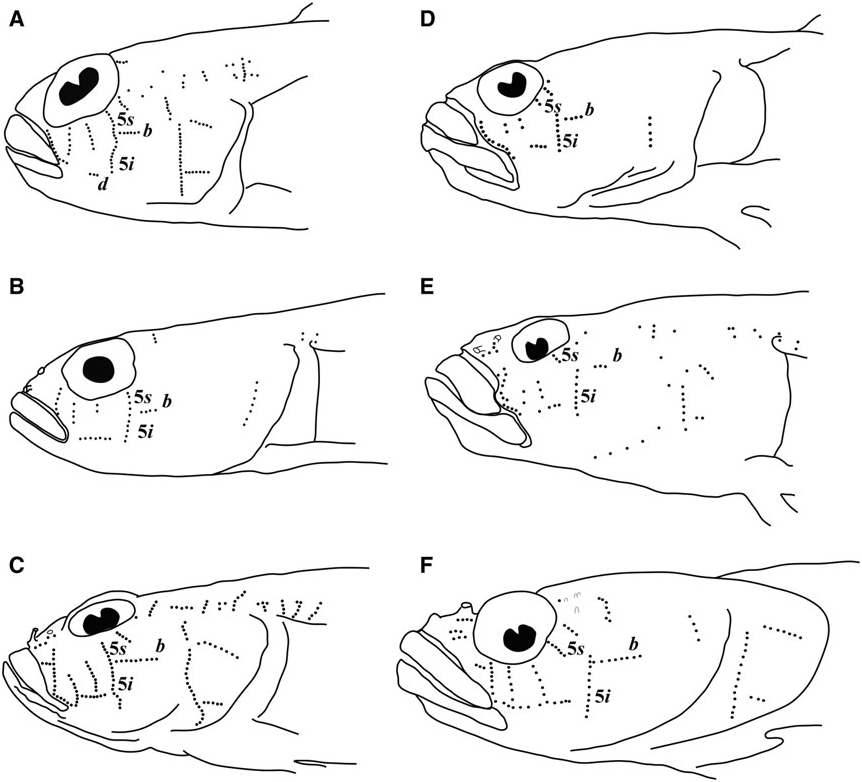
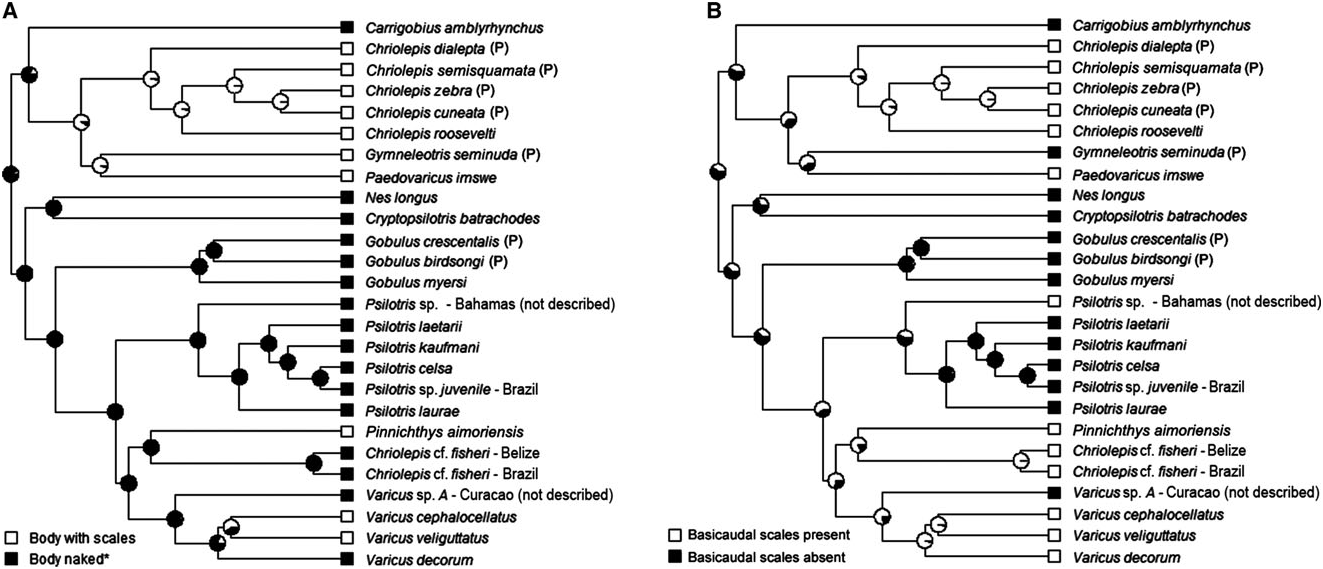
Pinnichthys is morphologically most similar to Chriolepis . The two genera share the plesiomorphic characters of two anal-fin pterygiophores inserted before the haemal spine, and cephalic lateralis pores absent. Pinnichthys and Chriolepis also possess body scales, although these may be have been independently gained in each group ( Fig. 7A View Figure 7 ). In general, Chriolepis is less heavily scaled than Pinnichthys , as most species of Chriolepis have fewer than 30 lateral scales rows (with Chriolepis dialepta occasionally possessing up to 35), and the scales never extend past the middle of the first dorsal fin (well short of this in most species). Chriolepis are also generally found in shallow water (<40 m), whereas Pinnichthys occur from depths of 70 m or more. Lastly, all Chriolepis have papillae rows 5i and 5s distinctly separate, with row 5s dorsal and well anterior of row 5i ( Fig. 3D – F View Figure 3 ), whereas Pinnichthys has rows 5i and 5s connected or nearly so (separated by the space of one papilla in Pinnichthys aimoriensis gen. et sp. nov.).
In addition to the type species, Pinnichthys aimoriensis gen. et sp. nov., the genus also includes the new species Pinnichthys saurimimica gen. et sp. nov., and three species previously assigned to Chriolepis : the eastern Pacific species Chriolepis atrimela , and the Atlantic species Chriolepis bilix and Chriolepis prolata . These three species all occur from deep reefs and have two anal-fin pterygiophores anterior to the first haemal spine, extensively scaled bodies, high anal-fin ray counts, and papillae rows 5i and 5s that are connected as a single row. The authors did not examine the papillae pattern for Chriolepis atrimela , but C. Thacker (pers. comm. 2015) at the Los Angeles County Museum of Natural History examined the holotype and confirmed the connection of 5i and 5s.
Etymology
The name Pinnichthys is formed from the roots pinna (Latin, feminine; fin) and ichthys (Latinized form of the Greek acronym ichthu s; fish). The name is given in reference to the high number of fin rays in the second dorsal fin and anal fin of all species in the genus.
PINNICHTHYS AIMORIENSIS VAN TASSELL & TORNABENE SP. NOV. THIONY’ S GOBY
FIGS 10 – 12 View Figure 10 View Figure 11 View Figure 12
Holotype
CIUFES 2414, 22.4 mm SL, male, plataforma Peroa , Espirito Santo, Brazil, – 19.577S – 39.264W, 70 m depth, 13 April 2012, T. Simon & H. T. Pinheiro. GoogleMaps
Paratypes
AMNH 265020 View Materials , 17.4 mm SL, male, collected at type locality, 13 April 2012 , T. Simon & H.T. Pinheiro; AMNH 265021 View Materials , 16.4 mm SL, female, cleared and stained, collected at type locality, 7 February 2014 , T. Simon and H.T. Pinheiro.
Diagnosis
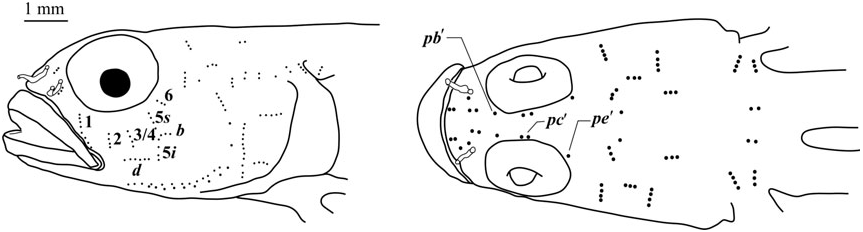
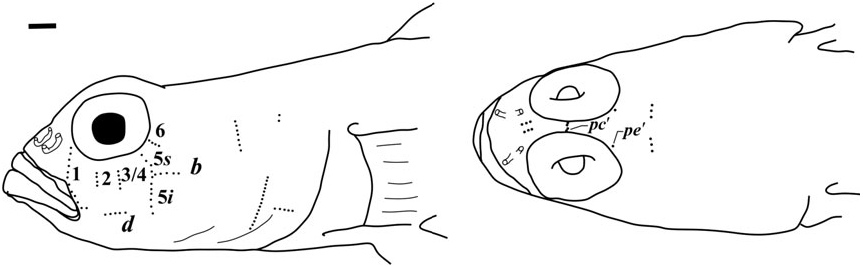
Side of body with 40 – 47 scale rows extending anteriorly to pectoral base; modified basicaudal scales present; first dorsal fin VII, without notably elongate spines, second dorsal I,10; anal fin I,10, rays fork only once near tips; pelvic fins well separated, no anterior frenum and no membrane connecting base of innermost rays; fifth pelvic-fin ray half the length of fourth and unbranched; pelvic-fin rays 1 – 4 branched, without fleshy tips; papillae rows 5s and 5i separate, lacking a papilla that would result in their forming a single continuous transverse row; interorbital papillae row pb’, pc’, and pe’ present; head and preopercle canals and pores absent; two anal-fin pterygiophores inserted anterior to haemal arch.
Description
Morphometric data are presented in Table 3.
Median and paired fins: First dorsal fin VII (3), spines 2 – 5 nearly equal in length, none notably elongate or filamentous; second dorsal fin I,10 (3), soft rays branch only once near tips; anal fin I,10 (3), soft rays branch only once near tips; pectoral-fin rays 19/18* (1), 18/18 (1) 18/? (1); pelvic fin 1,5 (3); pelvic fins well separated, lacking both anterior frenum and membrane connecting bases of innermost rays; fourth pelvic-fin ray longest, extending posteriorly beyond anus but falling short of anal-fin origin; first pelvic-fin ray branched once at midpoint; pelvic-fin rays 2 – 4 branched twice; fifth pelvic-fin ray unbranched, short, half length of fourth pelvic-fin ray; tips of pelvic-fin rays not ending in fleshy pads; caudal-fin rounded to slightly truncate; branched caudal-fin rays 14 (3); segmented caudal-fin rays 17 (3).
Scales: Trunk of body covered with ctenoid scales, extending anteriorly to pectoral base; 40 – 47 scales in lateral series (some scales missing anteriorly in holotype); eight or nine transverse scale rows; scales on abdomen cycloid (absent in female paratype); no scales on head and predorsal region; two modified basicaudal scales with enlarged ctenii present at dorsal and ventral margins of caudal-fin base.
Head: Jaw extending posteriorly to a vertical through the anterior margin of eye; anterior nare a short tube; posterior nare an opening with raised rim; no cephalic lateralis pores on head or preopercle; eyes 29.1 – 32.6% HL, dorsolateral, extending above profile of head, interorbital narrow, 7.0 – 10.7% HL; snout profile steep; operculum opening extending only length of pectoral-fin base; upper jaw with three or four rows at anterior, outer row enlarged and teeth spaced further apart; rows reduce to a single row, extending to posterior of premaxilla; lower jaw with four or five rows of conical teeth, outer two rows slightly larger, all teeth closely packed, tips slightly recurved; reducing to a single row extending to posterior of dentary.
Genitalia: Male urogenital papilla short, thin, and pointed; female urogenital papilla rounded and bulbous.
Colour in life ( Fig. 10 View Figure 10 ): Background colour of body and fins white; four large squarish brown spots along midline between level of anus and end of base of dorsal fin; first spot beneath pectoral fin, second spot below origin of second dorsal fin, third spot below middle of second dorsal fin, and fourth spot on caudal peduncle; an upper row of paler, more irregular, partly interconnected brownish yellow blotches along dorsal surface of body, extending forwards to under first dorsal fin, and partly interconnected with the mid-flank row; head pale with indistinct yellow marks on lower jaw, between eyes, and on nape; head behind eye to pectoral-fin base and abdomen with a yellowish pink hue; first dorsal fin translucent whitish at base, a wide yellow – brown stripe across middle of fin, distal quarter of fin white, outer half of fin heavily peppered with melanophores; second dorsal fin peppered with melanophores, with yellowish brown spots or broken stripes and white distal margin; caudal fin pale with yellow – brown spots and irregular vertical bars; anal fin dusky brown, with whitish basal stripe and distal margin; pectoral fins with no obvious pigmentation; pelvic fins with brownish base, distal three-quarters of fin white.
Colour in preservation ( Fig. 11 View Figure 11 ): Body with four dark blotches along lateral aspect of trunk, positioned as described above; in holotype, the pale space between each two spots with a narrow vertical bar of melanophores, bars lighter in intensity than squarish spots; body mostly pale below blotches; above blotches, four or five weakly separated dorsal saddles present that may connect to blotches; nape uniformly covered with dense peppering of melanophores; side of head with light scattering of melanophores; upper margin of pectoral-fin base with patch of dark melanophores, lower half unpigmented; pectoral rays unpigmented; first dorsal fin with dark lateral stripe across interspinal membranes, stripe with concentrated patches of melanophores over second, fourth, and fifth spines; second dorsal fin and caudal fin with several distinct, circular, dark spots on a pale background, spots on second dorsal of smallest paratype roughly arranged in three or four diagonal rows; anal fin with a pale horizontal stripe at base of rays, remainder of rays uniformly covered with dark melanophores, appearing uniformly grey to black; pelvic fins pale.
Sensory papillae ( Fig. 12 View Figure 12 ): A transverse pattern with abbreviated transverse rows; rows 1, 2, 3/ 4, 5s, 5i, and 6 present; rows 2 – 6 represented by three or four papillae; row 5s not reaching the level of row b and missing a complete connection to 5i by a single papilla; interorbital papillae row pb’, pc’, and pe’ present.
Vertebral skeleton: Dorsal pterygiophore formula 3 – 221110; two anal-fin pterygiophores inserted anterior to first haemal arch; second neural spine expanded and slightly spatulate at tip; hypurals 1 and 2 fused with hypurals 3 and 4 along at least half of their length; 27 vertebrae – 11 precaudal and 16 caudal.
Habitat: Collected from the seafloor near the Peroa natural gas platform. The substrate was predominately rhodoliths and other calcareous substrate.
Distribution: Known only from the margin of the continental shelf of Brazil off Espırito Santo.
Etymology: The species epithet aimoriensis is an adjective formed from the proper noun Aimores, an indigenous warrior people from the lands now belonging to the Brazilian states Espırito Santo, Bahia, and Minas Gerais. The Aimores people were virtually extirpated by European settlers during the Aimores War (1555 – 1673), and much of their native forest has been replaced by agriculture. The type locality for Pinnichthys aimoriensis gen. et sp. nov. is adjacent to the Peroa natural gas platform, and the nearby coastal region is facing rapid development from the petroleum industry and mining of rhodolith beds (carbonates) for agriculture, and may be under threat of losing biodiversity before it can be adequately studied and described. This situation is analogous to the loss of Aimores culture and the forest biodiversity that inhabited their native lands of the Central Brazilian coast.
The common name of Thiony’s Goby is given in honor of Thiony Simon. Thiony collected the type series of this species while scientific diving with friend and fellow ichthyologist Hudson Pinheiro. Together Thiony and Hudson pioneered scientific diving below 45 meters in the Central Brazilian coast, which contributed immensely to our knowledge of the regional biodiversity, and was key to discovering P. aimoriensis . Thiony, a promising young ichthyologist that in recent years stood out in the Brazilian ichthyological community, passed away at the age of 30 in a diving accident while this manuscript was in preparation.
Comparisons: Pinnichthys
aimoriensis gen. et sp. nov. can be distinguished from Pinnichthys bilix comb. nov. and Pinnichthys prolata comb nov. by having fewer lateral scale rows (40 – 47 vs. 30 – 27). Pinnichthys aimoriensis gen. et sp. nov. lacks the elongate first dorsal-fin spines of Pinnichthys bilix comb. nov. and the eastern Pacific Pinnichthys atrimela comb. nov. Lastly, Pinnichthys aimoriensis gen. et sp. nov. is distinguished from Pinnichthys saurimimica gen. et sp. nov. by having I,10 second dorsal-fin and anal-fin rays (versus I, 11 in Pinnichthys saurimimica gen. et sp. nov.).
PINNICHTHYS SAURIMIMICA GILMORE, VAN TASSELL & TORNABENE SP. NOV. LIZARDFISH GOBY
FIGS 13 – 16 View Figure 13 View Figure 14 View Figure 15 View Figure 16
Holotype
USNM 427228 View Materials , 55.4 mm SL, female, Cockburn Town , Riding Rock , San Salvador, Bahamas, 24.04833N, – 74.5375W, Johnson Sea Link I, Dive JLS-I 2024, 282 m depth, 3 May 1987, R. G. Gilmore & D. Liberatore. GoogleMaps
Diagnosis
Body scaled with 47 – 53 ctenoid scales, modified basicaudal scales present; first dorsal VII, no elongate filamentous spines present; second dorsal I,11; anal I,11; pectoral 20; pelvic fins I,5, separate, rays 1 – 4 branched between two and four times, fifth pelvic-fin ray long and unbranched, three-quarters length of fourth pelvicfin ray, no fleshy tips present on pelvic-fin rays; head and preopercle canals and pores absent; a transverse sensory papillae pattern with row 5s/5i connected, interorbital papillae pc’ and pe’ present; two anal-fin pterygiophores inserted anterior to haemal arch.
Description
Morphometric data are presented in Table 3.
Median and paired fins: First dorsal fin VII, spines 1, 2, and 3 successively longer, spines 4 and 5 longest, spines 2 – 5 extending slightly beyond membrane; second dorsal fin I,11, soft rays branch two or three times beginning midway along each ray; anal fin I,11, soft rays branch two or three times beginning midway along each ray; pectoral-fin rays 20; pelvic fin I,5; pelvic fins well separated, lacking anterior frenum, short membrane connecting fifth rays basally, fourth ray longest, extending threequarters distance to anus when extended posteriorly, rays 1 – 4 branched between two and four times, fifth long, unbranched, three-quarters length of fourth ray; tips of pelvic-fin rays not ending in fleshy pads; caudal fin oval; branched caudal-fin rays 14; segmented caudal-fin rays 17.
Scales: Trunk of body covered with ctenoid scales from beneath pectoral fin nearly to caudal-fin base, anterior scales with reduced ctenii; 53/47 (left/right) scales in lateral series; 11 transverse scale rows; cycloid scales on lateral and posterior portions of abdomen, scales absent on mid- and anterior portions of abdomen; a naked upper area from posterior end of first dorsal-fin base to upper pectoral-fin base; a naked lower area extending from abdomen near anus to lower pectoral-fin base; two modified basicaudal scales with enlarged ctenii present at dorsal and ventral margins of caudal-fin base.
Head: Jaw extending posteriorly to a vertical through anterior end of pupil; anterior nare an elongate tube, posterior nare a short tube, no flaps on edges; no cephalic lateralis pores on head or preopercle; eyes 30.9% HL, dorsolateral, extending above profile of head, interorbital narrow, 6.04% HL; snout profile steep; operculum opening extending length of pectoral-fin base; teeth in upper jaw arranged in four or five rows, outer row with enlarged widely spaced teeth continuing to near posterior of premaxilla, inner rows smaller and more numerous with slightly recurved tips; teeth in lower jaw in three or four rows, outer row with eight or nine large teeth, widely spaced, restricted to anterior of jaw, teeth in remaining rows smaller with slightly recurved tips.
Genitalia: Female (only known specimen) with short, rounded bulbous papilla, no melanophores present.
Colour in life ( Figs 13 View Figure 13 and 14 View Figure 14 ): Background colour of body and fins white; five large yellow – brown spots with dense concentrations of melanophores along lateral midline trunk, first (largest) under first dorsal-fin spines 3 – 6, second under anterior rays of second dorsal fin, third under second dorsal-fin rays 6 – 8, fourth equidistant between third and fifth spot, fifth (smallest) on caudal peduncle; four yellow – brown double bars stippled with melanophores along back positioned between the mid-lateral spots, crossing dorsal midline, bars lighter in colour than mid-lateral spots, single yellow bar on back before caudal peduncle; nape with three yellow bars, the first extending anterioventrally to the lower posterior margin of orbit, second nape bar extending to upper opercular margin, the third bar extending from near dorsal midline ventrally to upper pectoral base; yellow spot below eye near maxilla, other spots on cheek; iris bright yellow; prominent white pigment over both dorsal fins; caudal fin brilliant white, middle of anal fin white to base and distal margin, with black pigment along ventral one-third of fin; pelvic fins black; pectoral fins translucent white.
Colour in preservation ( Fig. 15 View Figure 15 ): Body light brown with five large spots just below lateral midline, positioned as described above; a small spot on posterior portion of caudal peduncle, near base of caudal-fin rays; a series of wide, double bars along dorsal portion of trunk, bars lighter in colour than body spots, first pair under first dorsal fin, second pair under second dorsal-fin rays 2 – 5, and third at posterior end of second dorsal; a single bar on caudal peduncle, ending at bases of caudal procurrent rays; dark pigment on nape where the three yellow bars are as described above; pectoral, first dorsal, second dorsal, and caudal fin translucent; pelvic fin with a few scattered melanophores; anal fin with a band of melanophores from middle to outer region of fin.
Comparisons: Pinnichthys
saurimimica gen. et sp. nov. is most similar to Pinnichthys bilix comb. nov. Both species have counts of I, 11 in the second dorsal and anal fins, and both occur off the Bahamas. Pinnichthys saurimimica gen. et sp. nov. differs from both Pinnichthys bilix comb. nov. and Pinnichthys prolata comb. nov. in having more lateral scale rows (47 – 53 vs. 30 – 37). Pinnichthys saurimimica gen. et sp. nov. also lacks the elongate first dorsal-fin spines that are present in Pinnichthys bilix comb. nov. and the eastern Pacific Pinnichthys atrimela comb. nov. Lastly, Pinnichthys saurimimica gen. et sp. nov. is distinguished from Pinnichthys aimoriensis gen. et sp. nov. by the presence of I,11 second dorsal-fin and anal-fin rays versus I,10, and in having slightly more lateral scale rows (42 – 47 in Pinnichthys saurimimica gen. et sp. nov. versus 47 – 53 in Pinnichthys aimoriensis gen. et sp. nov.).
| T |
Tavera, Department of Geology and Geophysics |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Nes
| Tornabene, Luke, Van Tassell, James L., Gilmore, Richard G., Robertson, David Ross, Young, Forrest & Baldwin, Carole C. 2016 |
PAEDOVARICUS
| Greenfield DW 1981: 269 |