Stenopelmatus, Burmeister, 1838
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4917.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:D89148CE-EE8A-46B8-8D8B-8F5790063FC4 |
|
DOI |
https://doi.org/10.5281/zenodo.4475890 |
|
persistent identifier |
https://treatment.plazi.org/id/03A4C420-8A62-FB5A-9B84-24DE1D0DFC9A |
|
treatment provided by |
Plazi |
|
scientific name |
Stenopelmatus |
| status |
|
Stenopelmatus View in CoL versus Ammopelmatus.
Morphological characters:
A. Rear leg tibial spines are generally longer in Stenopelmatus than Ammopelmatus , giving the impression of more spiny legs. Stenopelmatus exceptions would be those non-jumping taxa that includes S. perote , S. hondurasito , S. durango , and S. faulkneri .
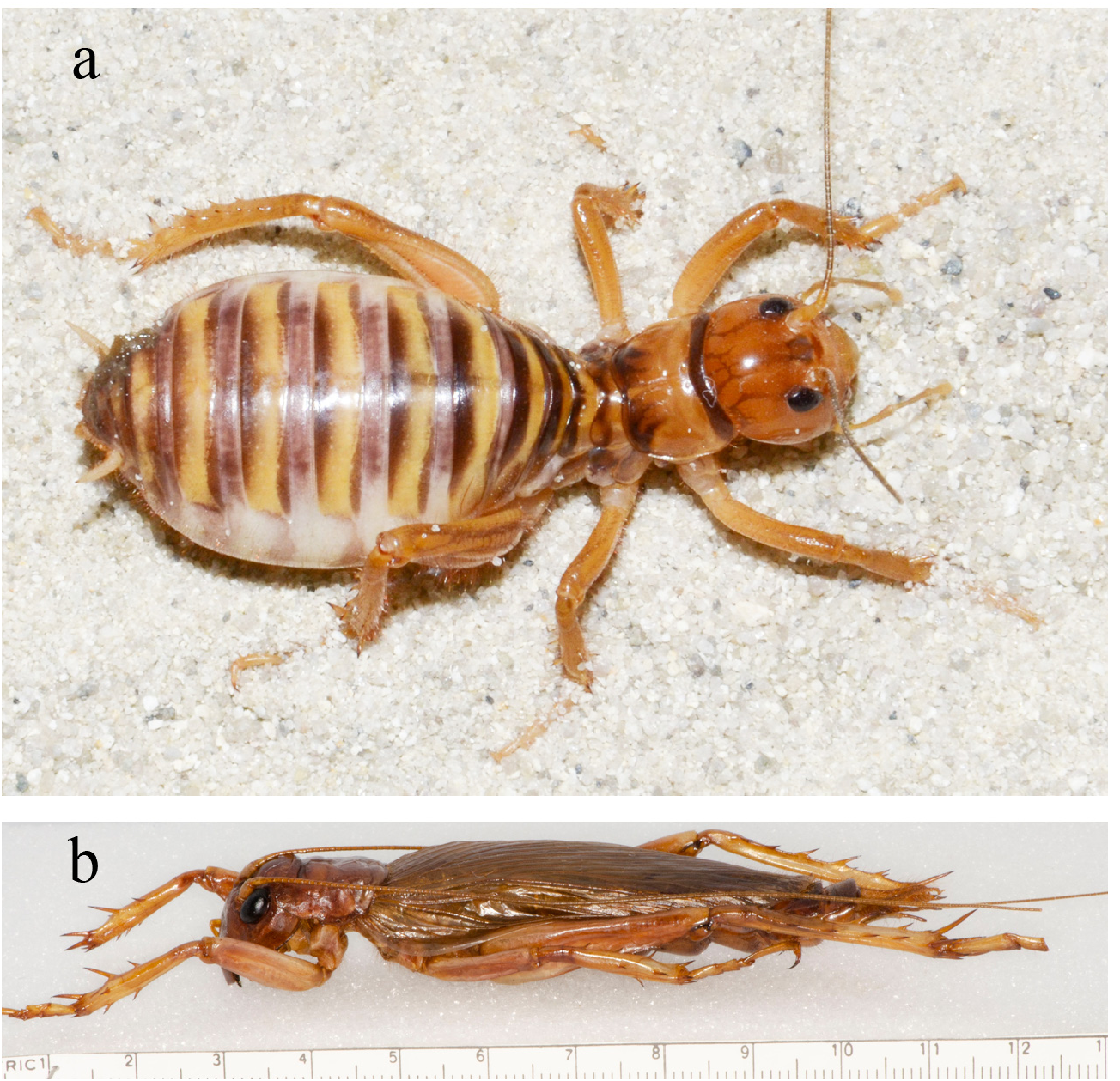
B. Body color. Almost all Stenopelmatus have solid, brightly colored abdomens including shiny black, reds, oranges and metallic sheens. Almost all species of Ammopelmatus have black-striped abdomens with body colors of plain tan and brown. Solid abdominal colors are found in A. mescaleroensis and A. mahogani but these are the exceptions. Black JCs, with “striped” abdomens ( S. histrio [last instar male, p. 45], S. toltecus [adult female, p. 103]), are of potentially little significance because the tergite plates can be separated, for example, by an egg-distended abdomen, resulting in a striped pattern, or can just be normal variation seen in a large series (see Fig. 108 View FIGURE 108 of S. perote , p. 68).
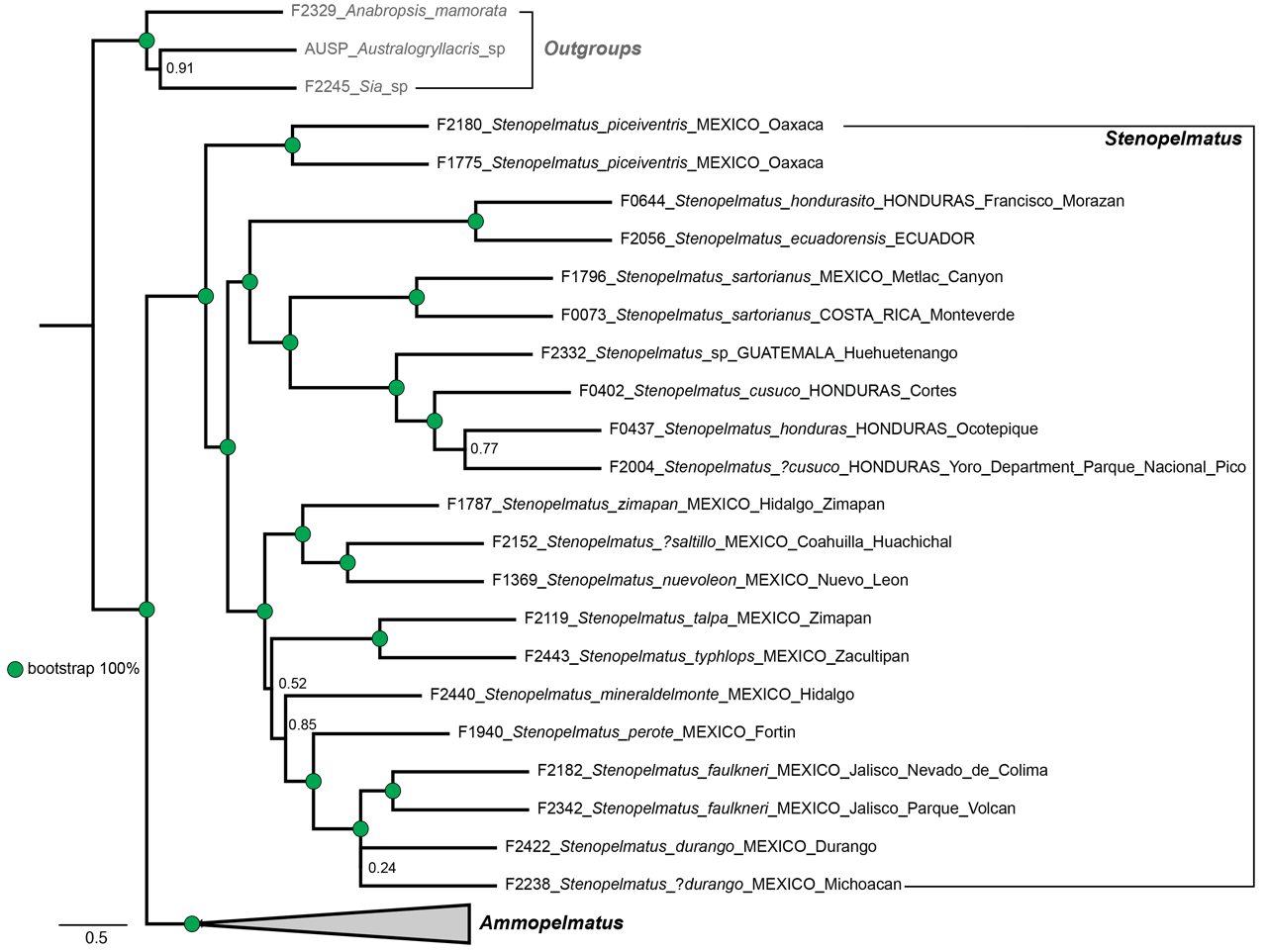
C. Adults with small or complete wings: Present in S. sartorianus , S. piceiventris , S. chiapas , S. sanfelipe . Absent in all Ammopelmatus . DNA demonstrates that this character has arisen more than once in Stenopelmatus , as S. piceiventris and S. sartorianus are not closely related in the phylogeny ( Fig. 10 View FIGURE 10 ).
D. The ovipositor in most Stenopelmatus species appears longer than in Ammopelmatus , and has a sharply recurved tip, easily appreciated, for instance, in S. ater (see Fig. 17 View FIGURE 17 ). In Ammopelmatus , the ovipositor curves more uniformly. The instar of female specimens is more difficult to identify in Stenopelmatus , perhaps because the ovipositor develops more slowly than in Ammopelmatus . It is unknown if both genera have the same number of molts.
E. The calcars on the rear leg tibia of all Stenopelmatus taxa are generally unspecialized while those calcars can be specialized in certain Ammopelmatus Groups (Weissman et al. in prep) along the lines of (1) scoop-like, in sand inhabiting species ( A. kelsoensis , A. muwu , and A. davewerneri ), (2) long in males of the Longispinus Group, and (3) thickened in males from the northwestern USA.
F. Megacephalism is almost unknown in Stenopelmatus while widespread, in both sexes, and within certain groups (i.e. the Fuscus Group) of Ammopelmatus . Large heads would be useful for those taxa that live in sandy habitats because JCs use their heads like trowels to dig through the sand: the bigger the scooper, the more efficient might be the forward movement. In contrast, the last thing that a JC that buries into rotten logs might want, would be a large head, perhaps explaining its rarity in Stenopelmatus .
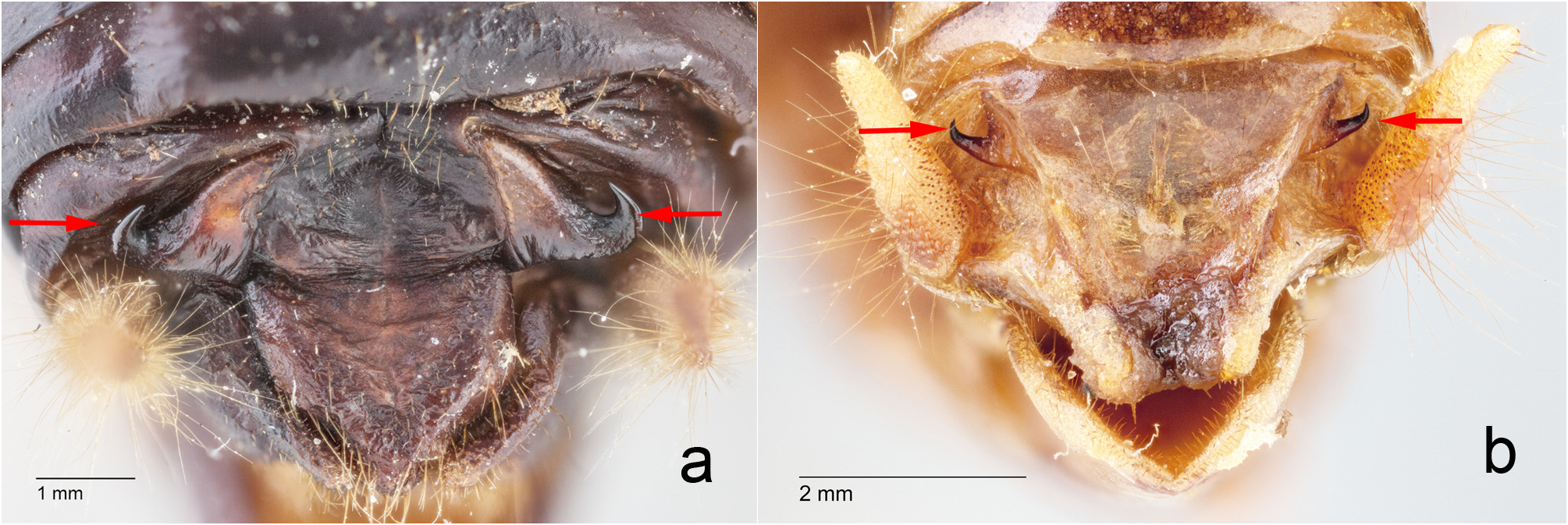
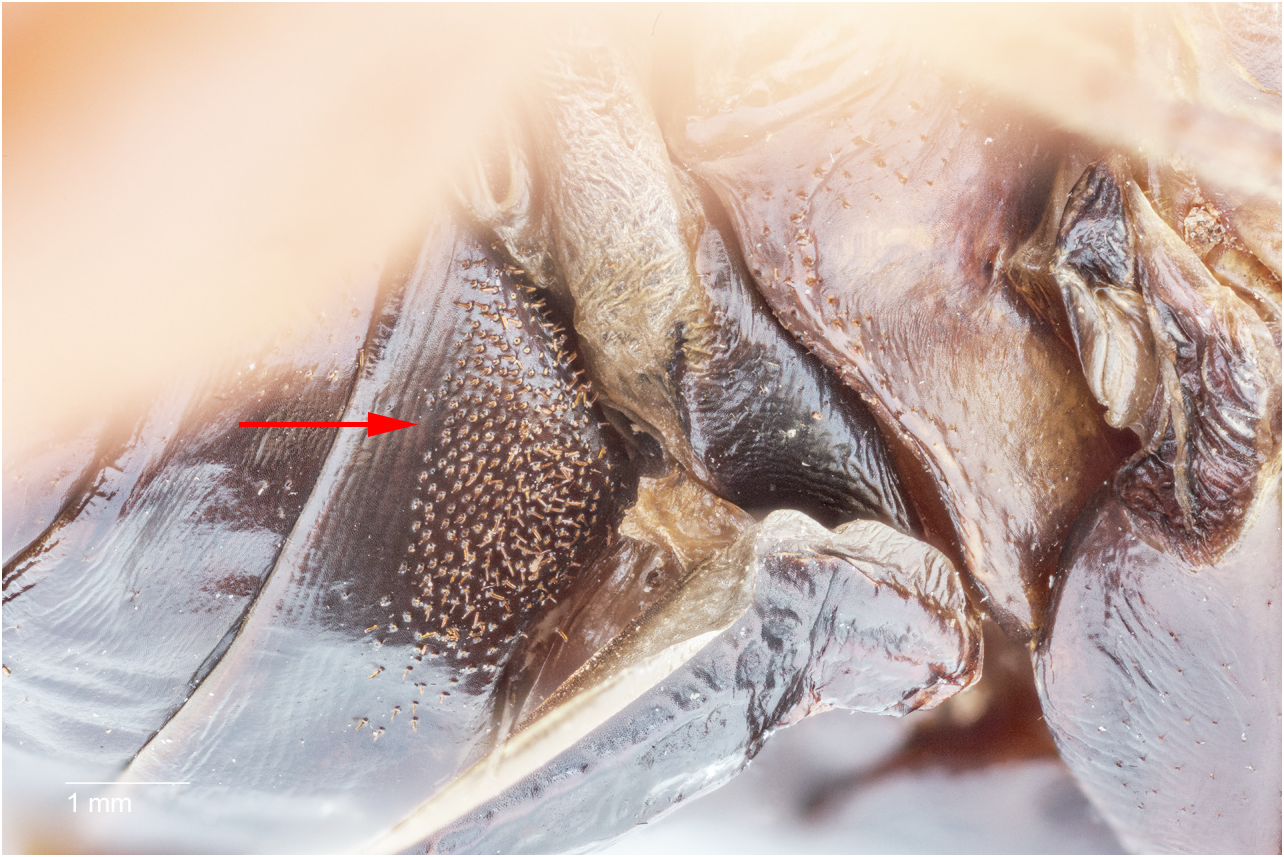
G. Hooks, on the dorsolateral margins of the supra-anal plate, in adult male Ammopelmatus , are typically visible with the naked eye, not hidden behind the cerci, and elevated ( Fig. 11b View FIGURE 11 ) away from the body such that running one’s finger across them results in a “catch.” In contrast, hooks in adult male Stenopelmatus ( Fig. 11a View FIGURE 11 ) can be smaller and darker such that 20X magnification is required to see them. They are usually appressed against the abdomen ( Table 2 View TABLE 2 ) along their entire length, such that there is no “catch” when running a finger across them. Weissman (2001b) determined that such hooks are necessary for successful mating in Ammopelmatus . Since S. sartorianus uses the same “bite-back” mating gymnastics (see p. 93), we suspect that these hooks are also necessary for successful mating in Stenopelmatus .
Ecological characters:
A. Habitats of Ammopelmatus species are generally dry, warm to hot, at lower elevations, frequently sandy desert, not forested, and never found in rotten logs during the daytime. Stenopelmatus is rarely in sandy habitats, frequently at higher elevations in cool, moist forests and cloud forests, and frequently in rotten logs during the daytime. F.T. Hovore (pers. comm. to DBW, 1996) believed that Stenopelmatus are more likely in rotten logs with passalid larvae, than scarab or cerambycid larvae, because such logs are older as passalid parents tend their larvae and make extensive tunnel systems.
B. Confirmation of A. is a good series of Stenopelmatus species collected under rocks, on ash, on sides of volcanoes, above 2,100m elevation (e.g. Paricutín Volcano, 22-vi-1955; Mt. Popocatépetl [see Figs 196 View FIGURE 196 , 197 View FIGURE 197 , p. 116 View FIGURE 116 , 117 View FIGURE 117 ]) above 3,000m, above tree line. Ammopelmatus are unknown from such habitats.
C. Stenopelmatus may be obligate carnivores, in contrast to omnivorous Ammopelmatus . Early on, we satisfied Stenopelmatus protein requirements, in the field, by feeding them passalid larvae collected from the rotting logs where we found them. Once back in the laboratory, we fed them commercially bought wax worm larvae ( Galleria sp.). Subsequently, we have successfully substituted fish food flakes (TetraFin) for the wax worm larvae.
D. Nymphs and adults of all species of Ammopelmatus are attracted to oatmeal trails. We have had similar good success with some of the small, black species of Stenopelmatus but no luck with any larger taxa and wonder if this might reflect their cannibalistic behavior.
Behavioral characters:
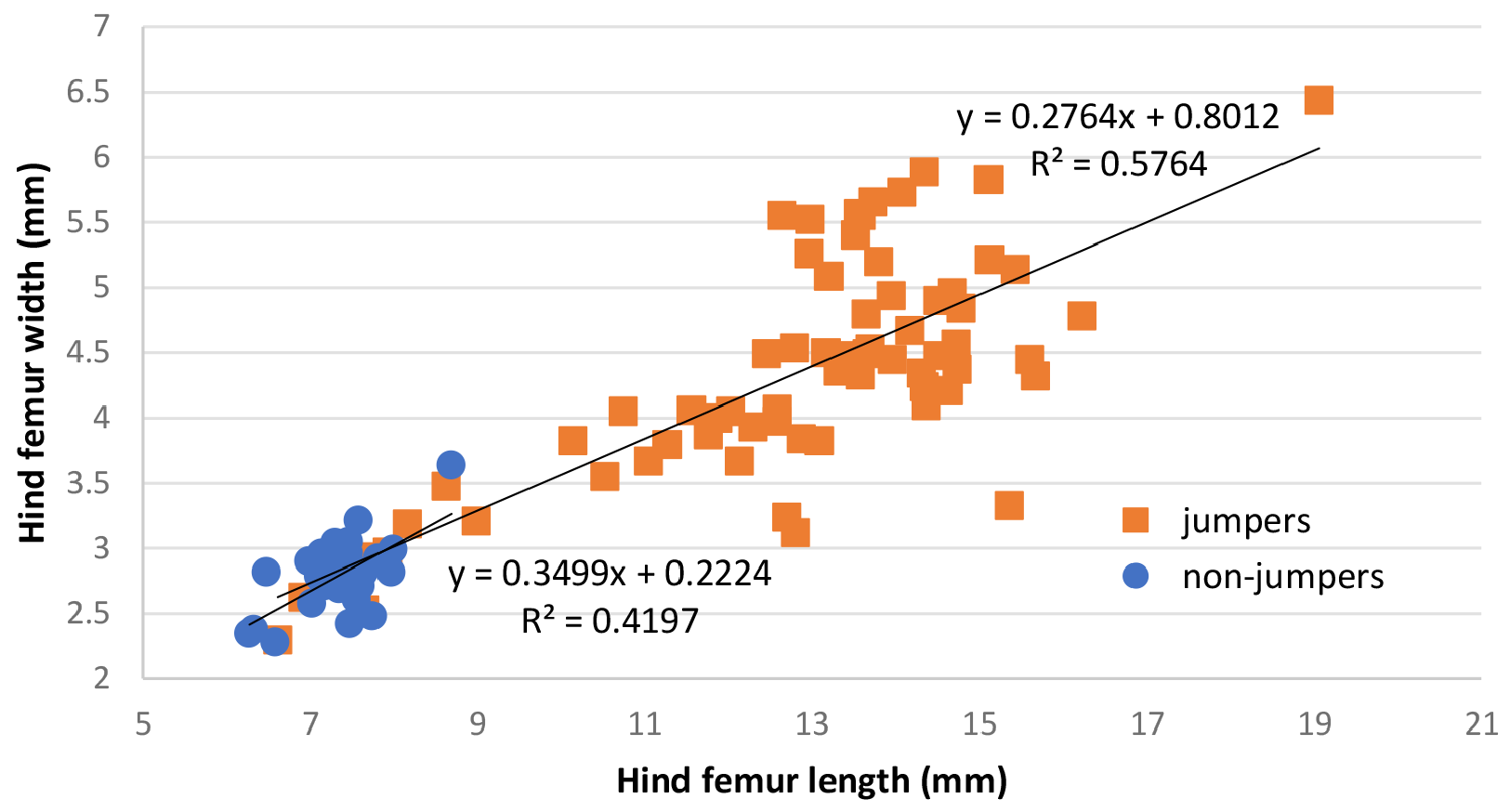
A. For the 16 named Stenopelmatus taxa for which we have first-hand experience ( Table 2 View TABLE 2 ), 10 species jump or hop as juveniles and adults. The 6 taxa that do not jump are all small (<25 mm). Of the 4 non-jumping taxa for which we have DNA, 3 of them ( S. perote , S. faulkneri , and S. durango ) are recovered together ( Fig. 10 View FIGURE 10 ). The 4 th nonhopper, S. hondurasito , is recovered closest to S. ecuadorensis in the ASTRAL and mtDNA analysis, but we don’t know if the latter hops or not. In the mtDNA tree ( Fig 9 View FIGURE 9 ), the clade with S. hondurasito is recovered closer to the other non-hopping taxa. Such hops/jumps can be from 6 to 15 cm in distance, and originate when both hind femurs are cocked and rapidly extended similar to the action seen when grasshoppers jump. In raising many thousands of Ammopelmatus over the years, we have never seen a single one jump. This trait is apparently the only character that is 100% diagnostic for Stenopelmatus , even if not all species do it. There is no relationship ( Fig. 12 View FIGURE 12 ) between hind femur length and hind femur width, perhaps analogous to the results of Lawler et al. (2019) who found no relationship between femur length: mass ratio in a grasshopper. On the other hand, the best jumper was S. sartorianus , a species with long, narrow femurs and tibias. These physical modifications appear to be similar in a jumping South Africa cockroach ( Picker et al. 2012).
B. Tree climbing was postulated by Weissman & Lightfoot (2007) for Stenopelmatus and subsequently confirmed by Gutiérrez-Rodríguez & Riverón (2018). We have never seen arboreal behavior in Ammopelmatus .
C. Antennae are constantly in motion in S. sartorianus , resembling that seen in an active pompilid wasp.
D. Virgin adults of both sexes of all species of Ammopelmatus generally drum well in captivity. Even in reluctant drummers, documentation was eventually successful. In many Ammopelmatus taxa, the drums are easy to hear at distances of 10m. In contrast, Stenopelmatus virgin adults are generally reluctant, much softer drummers. In fact, we were unable to document drumming in 3 species ( S. mineraldelmonte , S. piceiventris , S. hondurasito ) of Stenopelmatus despite repeated efforts with several virgin individuals of each species, and we speculate that not all Stenopelmatus taxa may drum. We wonder if this behavioral difference could be analogous to the decreased calling song stridulation seen in many, similarly nocturnal, New World tropical katydids, possibly as a result of high bat predation ( Belwood & Morris 1987)?
E. While a number of Ammopelmatus taxa possess a sex clarification drum ( Weissman 2001b), such is only known in one species of Stenopelmatus - S. perote (see p. 64).
Endophenotypic characters.
A. Fig. 2 View FIGURE 2 in Vandergast et al. (2017) shows 3 clades of New World JCs based on DNA sequencing. Clade 1 includes all Ammopelmatus taxa, most living entirely within the United States. Clade 2 includes all species of Stenopelmatus , all living south of the United States. Clade 3 includes S. piceiventris , a micropterous taxon with small fore wings. It is unknown if the apparently related S. sanfelipe , also with small fore wings, would be recovered near S. piceiventris , but we predict that it would. A 3 rd micropterous taxon, S. chiapas , was recovered (F2172) within Clade 2 but we caution that its DNA was old and its reliability is questionable and should be rechecked. Additional phylogenetic datasets presented here group S. piceiventris and all other Stenopelmatus separately from Ammopelmatus ( Fig. 9 View FIGURE 9 and Fig. 10 View FIGURE 10 ). Stenopelmatus piceiventris appears to be basal to all other Stenopelmatus .
B. Karyotype numbers vary in Ammopelmatus from 2nƋ=25 (most common) to 23 (next most common) to rarely 21 or 19. Only 4 species of Mexican JCs have been karyotyped: S. piceiventris has 2nƋ=27, and as it lies at the base of the tree, may represent the ancestral number of the subfamily. Stenopelmatus perote , S. typhlops , and S. zimapan all have 2nƋ=25.
Alphabetical listing of all Stenopelmatus Jerusalem cricket species, all from south of the United States’ border, discussed in this paper
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |