Barsine wangi Volynkin, Huang, Dubatolov & Kishida, 2019
|
publication ID |
https://doi.org/10.11646/zootaxa.4664.3.12 |
|
publication LSID |
lsid:zoobank.org:pub:BEAE2E75-F830-42A8-BE28-4C85214B34F0 |
|
persistent identifier |
https://treatment.plazi.org/id/E2FE874E-FC8C-4B20-857D-F3BD92B5A88F |
|
taxon LSID |
lsid:zoobank.org:act:E2FE874E-FC8C-4B20-857D-F3BD92B5A88F |
|
treatment provided by |
Plazi |
|
scientific name |
Barsine wangi Volynkin, Huang, Dubatolov & Kishida |
| status |
sp. nov. |
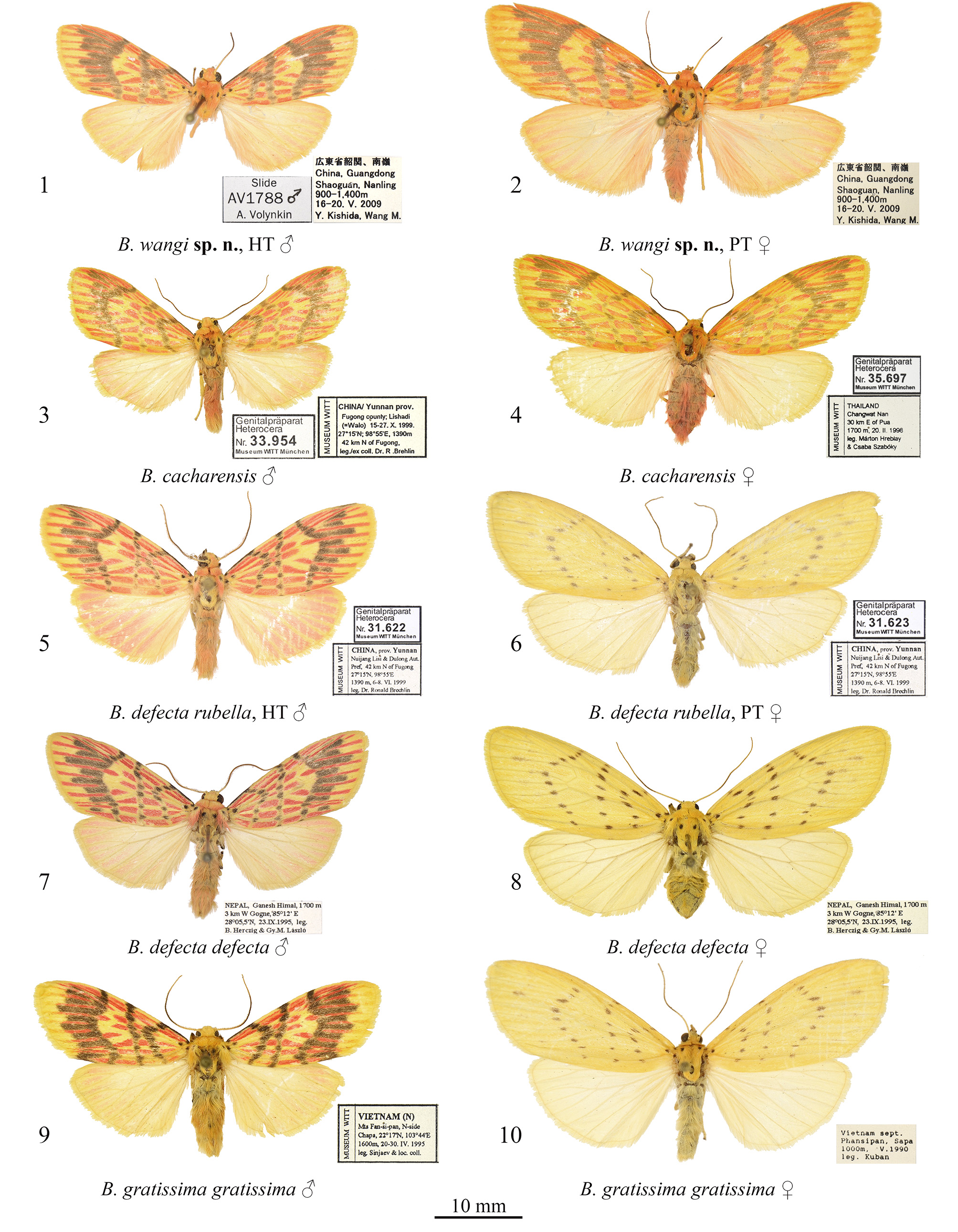
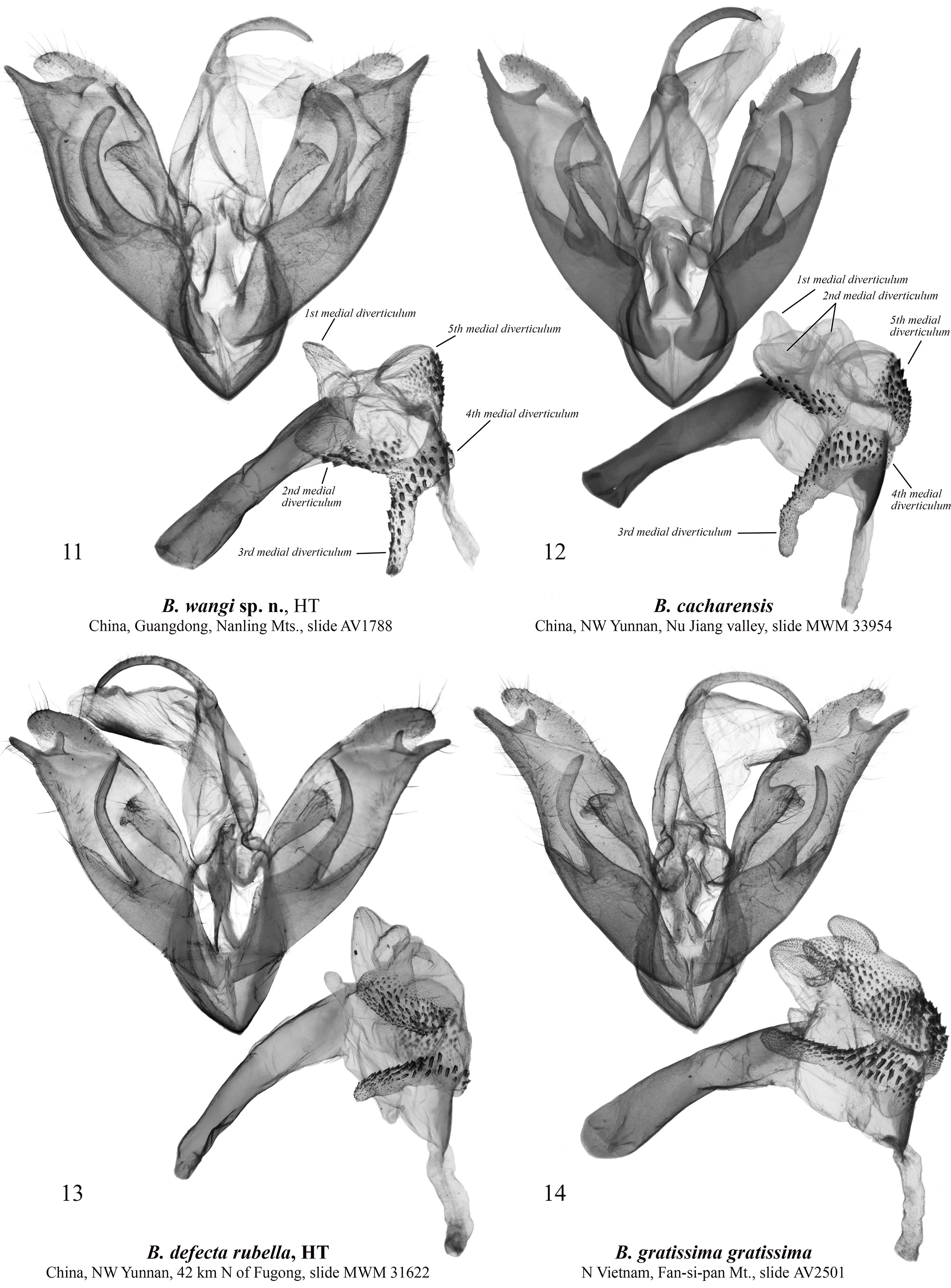
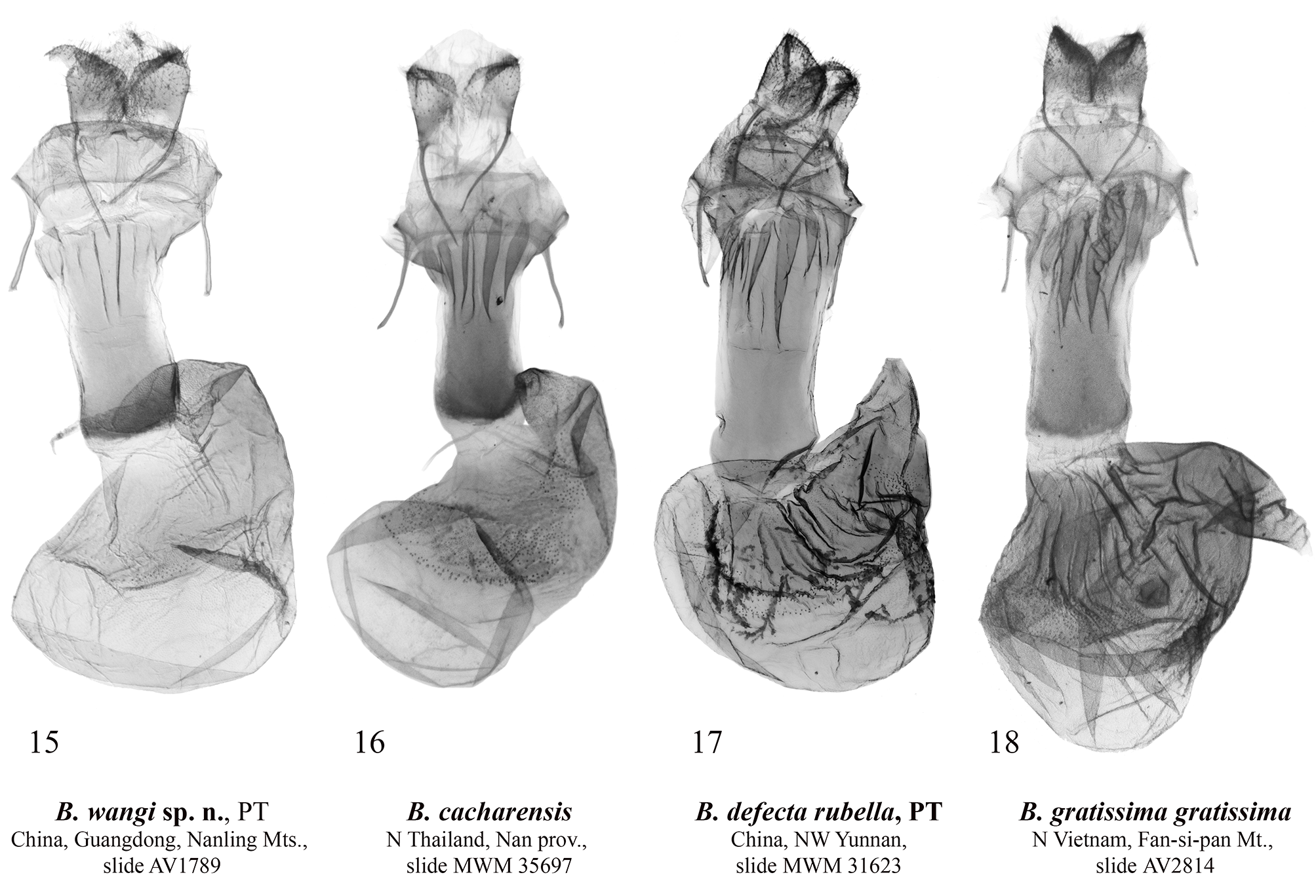
Barsine wangi Volynkin, Huang, Dubatolov & Kishida View in CoL , sp. nov. ( Figs 1, 2 View FIGURES 1–10 , 11 View FIGURES 11–14 , 15 View FIGURES 15–18 )
urn:lsid:zoobank.org:act:
Type material. Holotype ( Figs 1 View FIGURES 1–10 , 11 View FIGURES 11–14 ): ♂, “ China, Guangdong, Shaoguan, Nanling , 900–1400 m, 16–20.V.2009, Y. Kish- ida, Wang M.”, slide AV1788 ♂ Volynkin ( Coll. SZMN).
Paratypes: 2 ♀, same data as in the holotype, slide AV1789 ♀ Volynkin ( Coll. SZMN) ; 1 ♂, altitude 997m, 18.VIII.2003, Nanling, leg. Min Wang ( Coll. SCAU) ; 3 ♂, same locality and collector, but 5.V.2017 ( Coll. SCAU) ; 7 ♂, 3 ♀, same locality and collector, but 6.V.2017 ( Coll. SCAU) ; 2 ♂, same locality, but 20.V.2008, leg. He-shan Chen ( Coll. SCAU) ; 1 ♂, same locality and collector, but 10.VI.2008 ( Coll. SCAU) ; 4 ♂, 1 ♀, same locality, but 24.V.2014, leg. Hai-ling Zhuang & Lan-lan Huang ( Coll. SCAU) ; 3 ♂, 2 ♀, same locality, but 11.V.2018, leg. Fu-hong Wei & Si-yao Huang ( Coll. SCAU) ; 2 ♂, same locality and collectors, but 12.V.2018 ( Coll. SCAU) ; 1 ♂, 1 ♀, same locality and collectors, but 14.V.2018 ( Coll. SCAU)
.
Etymology. The species name is dedicated to Dr. Wang Min (South China Agricultural University, Guangzhou, China), one of the collectors of the holotype and of several paratypes.
Diagnosis. Male of B. wangi ( Fig. 1 View FIGURES 1–10 ) resembles externally males of B. defecta (especially its Chinese subspecies B. d. rubella Volynkin & Černý, 2017) ( Figs 5–8 View FIGURES 1–10 , 13 View FIGURES 11–14 , 17 View FIGURES 15–18 ) and B. gratissima ( Figs 9, 10 View FIGURES 1–10 , 14 View FIGURES 11–14 , 18 View FIGURES 15–18 ), but can be distinguished by its dark yellow forewing ground color (that is light yellow in B. defecta and B. gratissima ), more diffuse brownish-grey pattern elements, connected antemedial and medial lines (those are separated in B. defecta and B. gratissima ), shorter subterminal longitudinal stroke between the veins M1 and R5 (in B. defecta and B. gratissima that is the longest in a subterminal series), and principally different genitalia structure. Females of B. defecta and B. gratissima have reduced red pattern elements ( Figs 6, 8, 10 View FIGURES 1–10 ) and look clearly different from those of B. wangi ( Fig. 2 View FIGURES 1–10 ), which is most similar externally to that of the related B. cacharensis ( Fig. 4 View FIGURES 1–10 ) by the presence of red strokes on forewing, not connected antemedial and medial lines, and long longitudinal gray strokes of subterminal series, but differs clearly by its larger size, dark yellow forewing ground color (that is lighter in B. cacharensis ), darker red pattern elements, medial line angled at costa (that is angled medially in B. cacharensis ), postmedial line smoothly curved (that is angled medially in B. cacharensis ), longer and continuous dark strokes between veins in the subterminal area connected to the postmedial line (in B. cacharensis some of those are interrupted or not connected to the postmedial line), broader yellow outer part of the subterminal area, and pinkish hindwing (that is pale ochreous yellow in B. cacharensis ). The male genitalia of B. wangi ( Fig. 11 View FIGURES 11–14 ) are most similar to those of B. cacharensis ( Fig. 12 View FIGURES 11–14 ), but can be easily distinguished by their shorter uncus, significantly broader and slightly shorter valve, much broader basal saccular process, much more robust distal saccular process with broader lobes, narrower vesica with unilobate 2 nd medial diverticulum (that is bilobate in B. cacharensis ), larger cornuti on the 4 th medial diverticulum, and smaller cornuti on the 5 th medial diverticulum. The female genitalia ( Fig. 15 View FIGURES 15–18 ) differ from those of B. cacharensis ( Fig. 16 View FIGURES 15–18 ) by their less folded antrum, broader ductus bursae and slightly longer appendix bursae.
Description. Adult ( Figs 1, 2 View FIGURES 1–10 ). Forewing length 15–18 mm in males ( 17 mm in the holotype), and 21–23 mm in females. Antennae of both sexes ciliate, ochreous basally and dark brown medially and distally. Head orange red; thorax dark yellow, with two black spots; patagia orange red; tegulae dark yellow with a black spot medially and broad orange red border; abdomen pale pink. Forewing ground color dark yellow; subbasal and medial areas with bright red longitudinal spots on veins; pattern elements dark grey. Subbasal spot as a dot; transverse lines broad, with diffuse margins; antemedial line strongly angled in the cell; medial line slightly curved and angled inwards at costa, connected to the antemedial line in males; postmedial line smoothly curved; subterminal area with long dark grey longitudinal strokes of various length between veins connected to the outer margin of postmedial line, and bright red strokes along veins. Cilia dark yellow. Hindwing pale yellow with pink suffusion between veins; cilia dark yellow. Male genitalia ( Fig. 11 View FIGURES 11–14 ). Uncus narrow, laterally flattened, curved, medially broadened, with claw-like tip; tuba analis broad. Scaphium narrow, weakly sclerotized. Tegumen moderately broad, shorter than valve. Juxta weakly sclerotized, X-shaped. Vinculum short but robust, V-shaped with slightly curved lateral margins. Valve broad, massive. Medial costal process broadly trigonal with curved outer margin. Distal costal process short, pimple-like. Distal membranous lobe of valve large, oblique. Sacculus broad, its basal process robust, broad, curved dorsally, apically rounded, reaches the distal costal process. Distal saccular process broad, bilobate, its dorsal lobe broadly trapezoidal; distal lobe elongated, trigonal, apically tapered. Aedeagus elongated, narrow, almost straight. Vesica membranous, short, with several diverticula: 1 st medial diverticulum elongated, conical, its distal half weakly granulated; 2 nd medial diverticulum broadly trigonal with rounded apex, its outer surface with narrow cluster of small trigonal cornuti; 3 rd medial diverticulum long, covered with small trigonal cornuti; 4 th medial diverticulum globular, covered with small trigonal cornuti; 5 th medial diverticulum broadly trigonal with rounded apex, its outer surface with broad cluster of small trigonal cornuti of various size; basal diverticulum absent; basal plate of vesica ejaculatorius broad, trigonal, heavily sclerotized. Female genitalia ( Fig. 15 View FIGURES 15–18 ). Papillae anales broad, trapezoidal; apophyses long, thin, of equal length; ostium bursae broad; antrum broad, funnel-like, with several narrow longitudinal folds. Ductus bursae elongated, dorso-ventrally flattened, sclerotized, its lateral margins more weakly sclerotized than medial part. Corpus bursae sack-like, its posterior section weakly sclerotized; medial sections weakly sclerotized, with narrow cluster of very small denticles; anterior section broadened, membranous, with area of weak granulation posteriorly. Appendix bursae weakly sclerotized and granulated, short, conical, situated postero-laterally, directed inwards.
Distribution. The new species is currently only known from the Nanling Mountains in northern Guangdong, China.
| SZMN |
Siberian Zoological Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Arctiinae |
|
Genus |