Phymastichus holoholo, Honsberger, David N & Wright, Mark G, 2022
|
publication ID |
https://doi.org/10.11646/zootaxa.5116.1.5 |
|
publication LSID |
lsid:zoobank.org:pub:326AFAC4-6A8E-4F40-88F8-7D626210D810 |
|
DOI |
https://doi.org/10.5281/zenodo.6366462 |
|
persistent identifier |
https://treatment.plazi.org/id/B21025E9-348A-4791-AE74-BCCC5733253B |
|
taxon LSID |
lsid:zoobank.org:act:B21025E9-348A-4791-AE74-BCCC5733253B |
|
treatment provided by |
Plazi |
|
scientific name |
Phymastichus holoholo |
| status |
sp. nov. |
Description of Phymastichus holoholo sp. nov.
Diagnosis: Among described genera of Tetrastichinae, Phymastichus holoholo falls within the genus Phymastichus as redescribed by LaSalle (1995) given the characters of a swollen parastigma on the forewing; male scape lacking a sensory plaque; scutellum without submedian or sublateral sulci, and anterior pair of setae closer to anterior margin of scutellum than posterior setae (though only slightly in this species); mesoscutum without median sulcus.
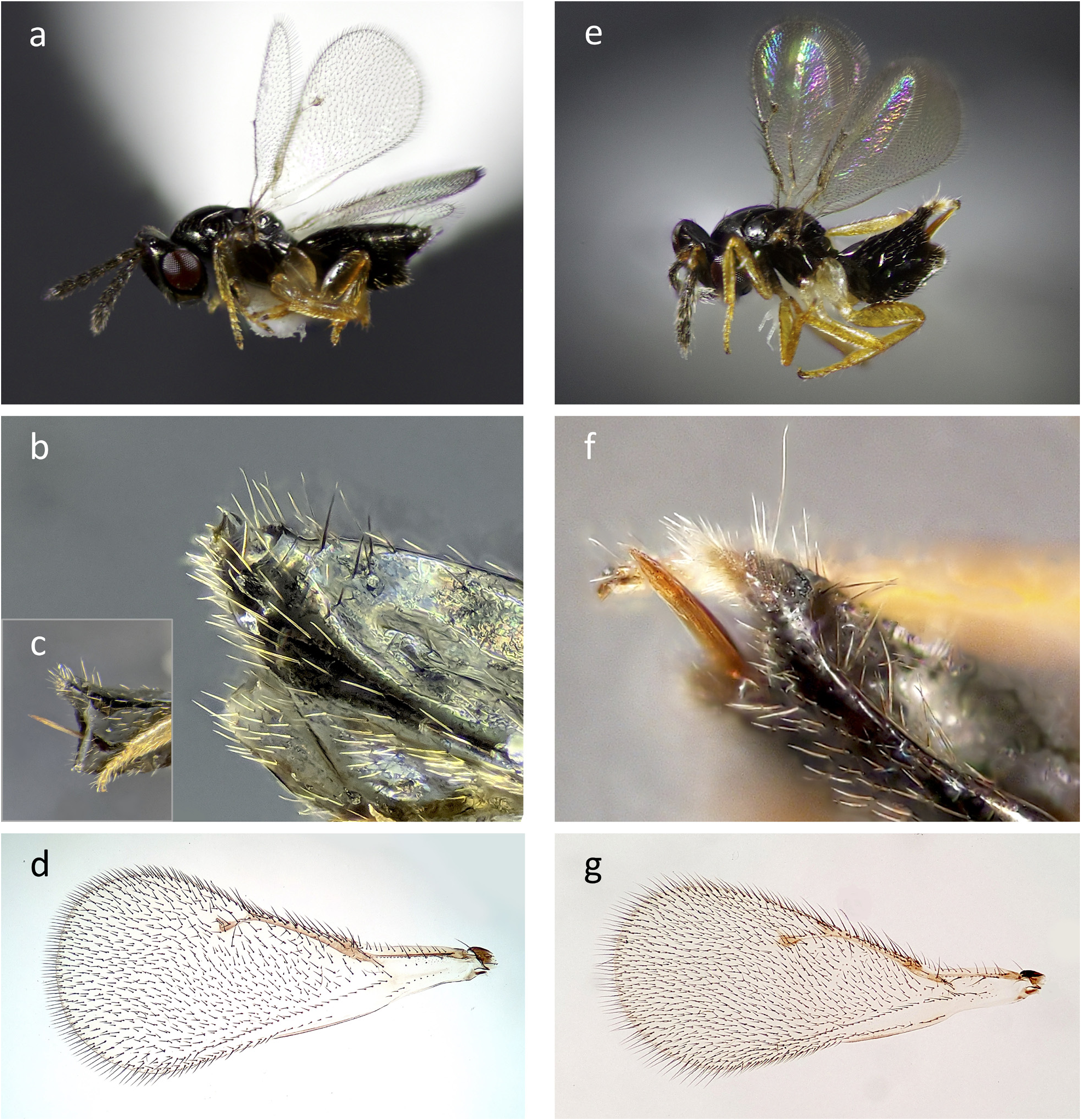
Overall, females of this species can be distinguished within Tetrastichinae by the combination of the enlarged parastigma and hyaline forewing membrane ( Figs 1d View FIGURE 1 ; 3a,d View FIGURE 3 ); a step-like carina on the syntergum, giving the apex of its gaster a crimped appearance dorsally, with sparse setae on either side of the carina and lacking a distinct tuft of setae posterior to the carina ( Figs 1a,b View FIGURE 1 ; 2a,b View FIGURE 2 ; 3h View FIGURE 3 ); the subcubital and cubital setal lines of the forewing distinctly separated until near the apex of the trailing edge of the wing ( Figs 1d View FIGURE 1 ; 3a,d View FIGURE 3 ); coloration and general habitus ( Figs 1a View FIGURE 1 ; 2 View FIGURE 2 ). They parasitize the adult stage of some Xyleborus beetles and potentially other Scolytinae as well.
Differential diagnosis: Among the other described species of Phymastichus , P. holoholo females can be distinguished from P. coffea by a strong step-like carina on the syntergum ( Figs 1b View FIGURE 1 ; 3h View FIGURE 3 ) (this step-like carina absent in P. coffea ); meso- and metacoxae lighter in color than the dark-brown to black body, metacoxae yellow to translucent white ( Figs 1a View FIGURE 1 ; 2a View FIGURE 2 ) (all coxae of similar color to the dark-brown to black body in P. coffea ).
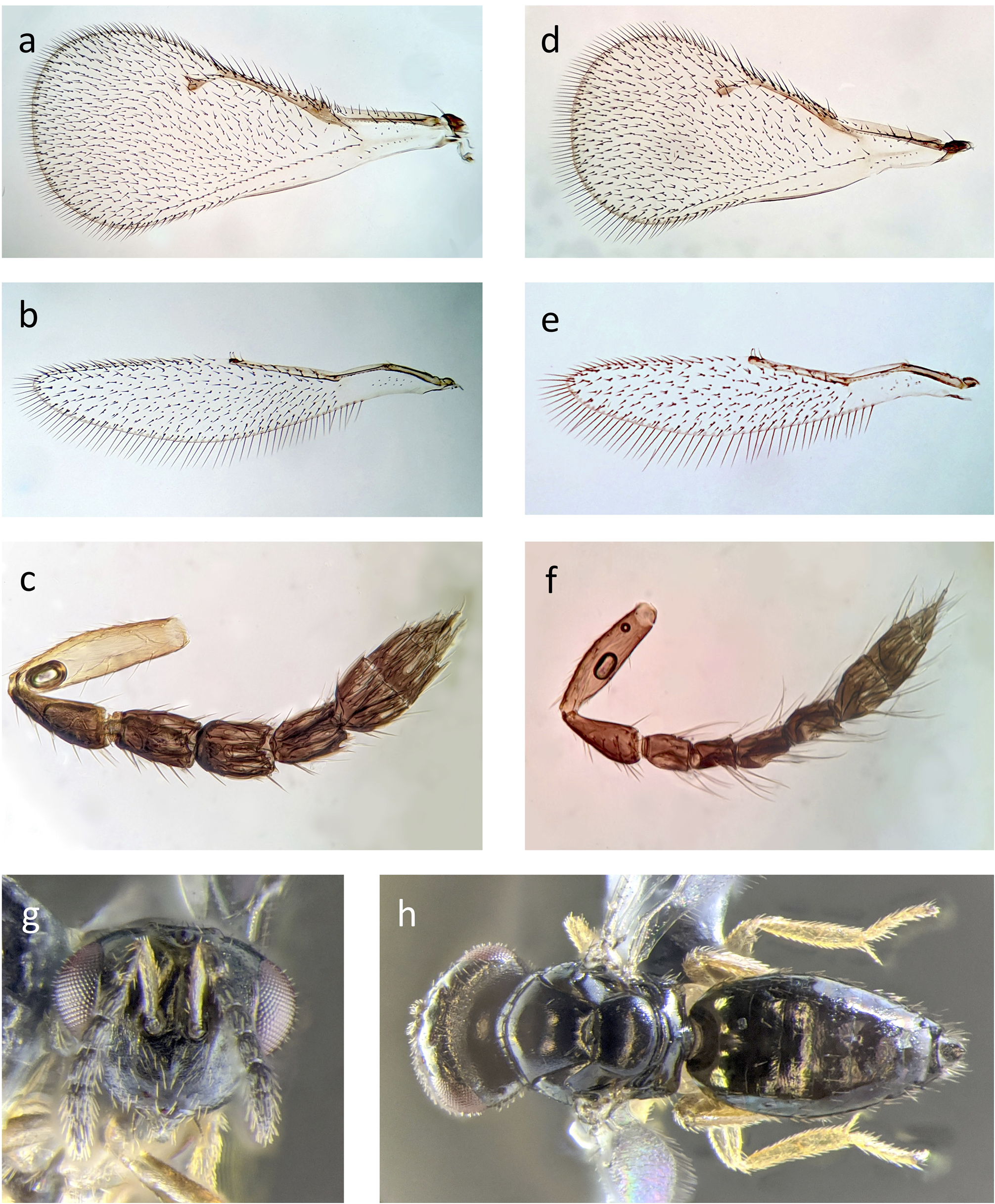
Phymastichus holoholo females can be distinguished from P. xylebori by the smooth cuticle anterior and posterior to the carina on the syntergum, and few setae medially posterior to the carina ( Figs 1a,b View FIGURE 1 ; 2a,b View FIGURE 2 ; 3h View FIGURE 3 ) (scale like sculpture anterior to the carina on the syntergum, and posterior to the carina, a dense tuft of light colored setae extending past the end of the gaster in P. xylebori ( Fig. 1e,f View FIGURE 1 )); ovipositor thin and needle-like, usually retracted in dry or alcohol preserved specimens and not visible ( Figs 1a–c View FIGURE 1 ; 2a View FIGURE 2 ) (ovipositor distinctly laterally flattened in P. xylebori , and usually visible in dry or alcohol preserved specimens ( Fig. 1e,f View FIGURE 1 )). Both sexes of P. holoholo can be distinguished from P. xylebori by the trailing edge of the forewing, where the cubital and subcubital setal lines of the forewing remain distinctly separate and with no setae between them until approximately the apex of the trailing edge of the wing ( Figs 1d View FIGURE 1 ; 3a,d View FIGURE 3 ) (the cubital and subcubital lines become difficult to distinguish at approximately half the length of the forewing, near the distal apex of the retinaculum in P. xylebori ( Fig. 1g View FIGURE 1 )).
Female ( Figs 1a–d View FIGURE 1 ; 2a–c View FIGURE 2 ; 3a–c,g,h View FIGURE 3 ):
Length: 1.2–1.5 mm ( Holotype 1.3 mm). Size in general correlates with the size of the beetle host. The length range recorded here is for wasps emerging from the beetles Xyleborus ferrugineus (Fabricius) , Xyleborus affinis Eichhoff , and Xyleborus perforans (Wollaston) , the currently confirmed hosts. If this wasp uses hosts other than these beetles, its size is expected to vary according to the size of the beetle, and may fall outside this range.
Head: Cuticle dark brown and shiny, with lightly reticulate sculpture visible under high magnification. Toruli located just above level of lower edge of eyes, scape reaching approximately to the level of the anterior ocellus. Scrobal depression shallow, its lateral edges smoothly rounded. Scrobal depression an inverted heart shape, widest at the toruli and tapering to a point below the anterior ocellus, a small triangular shaped interscrobal crest protruding between the toruli and reaching to approximately the level of the top of the toruli. The floor of the scrobal depression evenly rises to a shallow medial elevation that runs from the apex of the depression to the interscrobal crest. Frontofacial sutures consisting of two thin white lines, beginning at the outer margin of the two posterior ocelli and converging at the apex of the scrobal depression. Note that in air dried specimens much of the face from above the clypeus to the ocelli, including the entire scrobal area, is almost always collapsed and many of these characters are obscured. Antennae with scape, pedicel, a single anellus, 3 funicular segments, and a 3-segmented clava. Pedicel and flagellomeres apical of the anelli dark brown, similar in color to body, the scape lighter in color. The anellus typically appears more yellow than the rest of the antennae, and the pedicel can also be lighter in color than the flagellum in some individuals. Flagellum setose, and with sensilla including four apically converging terminal sensillar projections on the last segment of the clava that can be seen under high magnification. Setae on the pedicel and flagellum angled about 30–45 degrees from the apical direction of the antenna, and approximately 1/2 to 2/3 as long as the funicular segments are wide. Scape also setose, though the setae are shorter and thinner than on the pedicel and flagellum, and with a flattened area ventrally to receive the rest of the antenna when folded. Funicular segments longer than wide. Ratio of the length of the antennal segments, not including the anellus, approximately Scape: Pedicel: F1: F2: F3: F4: F5: F6 = 2.2: 1.2: 1.0: 0.9: 1.0: 0.8: 0.7: 0.5. Eyes red to black with sparse, very small, short setae. Ocelli positioned in the form of an obtuse triangle, posterior ocelli closer to compound eyes than to each other, cuticle of the ocellar triangle raised relative to the rest of the vertex. Setae short on vertex, longer and of similar density on the face, shorter and of higher density on occiput. Margins of clypeus not delimited by a suture, but its upper corners with an anterior tentorial pit on each side. Malar sulcus complete. Mandibles tridentate and symmetrical, the ventral tooth pointed but not sharply, the middle tooth broadly rounded, the dorsal tooth differentiated from the overall curvature of the inner edge of the mandible by a small notch.
Mesosoma: Cuticle, with the exception of the legs (see below) and tegula which are typically translucent yellow, dark brown to black and shiny with light reticulate sculpture visible under high magnification. Pronotum with a transverse row of posterior pointing setae near the border with the mesoscutum, except near the midline which is free of setae, the first one or two setae lateral of the midline longer than the others. Additional setae often present anterior to this row. Mesoscutum lacking a medial sulcus on cuticle surface, and with three adnotaular setae, the anterior one approximately even with most anterior reach of the axillae, the two posterior setae near the scutellum, close together, and longer than the anterior seta. Mesoscutum mesal of notauli otherwise free of setae. Mesoscutal lateral lobe with setae pointing in various directions, of similar density to those on the posterior region of the pronotum. Scutellum with two pairs of setae near the lateral margins of the disk, one pair slightly anterior of the middle and the other pair near the posterior margin, a pit also present between the anterior and posterior setae. Lateral to the scutellar disk, the scutellum transitions smoothly to the impressed axillula where the cuticle is slightly wrinkled but lacks the set of distinct carinae present in P. xylebori . Propodeum strongly indented posteriorly to receive the small petiole, its margin also slightly concave where it comes up against the dorsellum. Median propodeal carina present but wide and somewhat inconspicuous. Plicae absent, callus with setae posterior and lateral to the spiracle, including on the convex region where the inclination of the sclerite becomes almost vertical.
Legs: Yellow to translucent yellow below coxae, though in some individuals the femora also have a black tint. Procoxae dark brown to black, similar in color to the body. Meso- and metacoxae translucent yellow, often lighter in color than the rest of the leg. Mildly curved tibial spurs on all legs. Tarsi and tibiae setose, femora with some setae but largely bare. Pro- and mesofemora of similar shape and thickness, metafemur substantially thicker. First through third tarsal segments subequal in length in all legs, fourth not including the pretarsus slightly longer. Tarsal claws brown in color, more robust in the mid and hind legs than in the foreleg.
Wings: Forewing: Parastigma slightly to distinctly swollen, as is characteristic for this genus. Cubital and subcubital setal lines join near the apex of the trailing edge of the wing, though the non-setose region between them becomes narrower distal to the retinaculum. Postmarginal vein protrudes only slightly if at all from the junction of the marginal and stigmal veins, and is much shorter than the stigmal vein. Marginal and submarginal veins subequal in length. Submarginal vein usually with three strong dorsal setae, and other smaller setae on the membrane anterior to the vein. Marginal vein with many strong setae. Veins somewhat uniformly yellow-brown in color, except for the stigmal vein which is transparent basal of the stigma. Stigma yellow-brown, and uncus present with four sensilla. Membrane subhyaline, apical of the small speculum and anterior to the cubital setal line covered approximately evenly over its surface with short setae. These setae are uniformly produced on the dorsal surface of the wing. On the ventral side, in the apical region of the wing they are similar in length to those on the dorsal side, but become strongly reduced in the medial and basal regions, especially closer to the trailing edge.
Hindwing: Membrane hyaline, the density and size of setae on the dorsal surface similar to that of the forewing; setae on the ventral surface reduced to dark spots. Trailing edge with long marginal setae consistently about 2/5 the maximum width of the hindwing. Veins yellow-brown in color. Three hamuli spaced closely together extend forward from the venation near its apex.
Gaster: Cuticle shiny dark brown to black, with somewhat reticulate sculpture visible under high magnification. Each gastral segment except the last with a transverse line of setae, setae longer and more upright on posterior segments. Gaster with hypopygium often distinct in dried specimens, extending near to the end of the gaster, cavernous posteriorly. The hypopygium is, however, not clearly distinguished in some individuals, and the shape of the gaster as a whole can vary. Relative to the rest of the gaster, setae increase in density on the hypopygium around the margins of the cavity and on the ventral part of the gaster posterior to the hypopygium. Syntergum with distinct step-like carina, similar to that of P. xylebori , giving the very end of the gaster a crimped appearance. Cerci located directly posterior to the carina. Anterior to the carina, the cuticle is smooth, without scale-like sculpture as in P. xylebori , and medially free of setae. Posterior to the carina, the cuticle is also smooth and shiny, the same color as the rest of the gaster and with at most a few short setae medially, free of long setae other than those emerging from the cerci. Ovipositor almost always retracted and only rarely projecting in dry or alcohol preserved specimens, but if visible, straight and needle-like, not especially flattened laterally in cross section as it is in P. xylebori .
Male ( Figs 2d–f View FIGURE 2 ; 3d–f View FIGURE 3 ): Similar in overall shape and coloration to female, but with the following differences:
Size: 0.7–0.8 mm long ( Allotype 0.75 mm). Typically much smaller than the females, and always smaller than a female emerging from the same host beetle.
Head as in female except: Antennae substantially thinner than in the female and with one more flagellar segment, consisting of scape, pedicel, a single anellus, and 7 flagellomeres, the last 3 of which form a clava. Ratio of the length of the antennal segments, not including the anellus, is approximately Scape: Pedicel: F1: F2: F3: F4: F5: F6: F7 = 2.9: 1.8: 1.0: 1.0: 1.1: 1.3: 1.2: 1.1: 0.8. Antennae setose and with sensilla, setae longer than the width of the funicular segments. Last antennal segment ends in a single terminal sensillum. As in the two other known species of Phymastichus , and unlike all other known members of Tetrastichinae, males lack a ventral sensory plaque on the scape.
Mesosoma as in female except: Legs with all coxae dark brown at least basally and medially, of similar color to the body, often lighter apically; femora and tarsi also typically somewhat darker than in the female, though this varies among individuals. Wings similar in overall structure to female except forewing with parastigma somewhat swollen but variable and typically not as swollen as in the female; submarginal vein often with only two long setae in addition to shorter setae.
Gaster: Overall somewhat oval in shape in dorsal view; setae in a similar pattern though overall less setose than in females; last tergite free of setae except for those emerging from the cerci.
Repositories
UHIM—University of Hawaiʻi Insect Museum, Honolulu, Hawaiʻi, USA
BPBM—Bernice Pauahi Bishop Museum, Honolulu, Hawaiʻi, USA
NHMUK—The Natural History Museum, London, UK
Holotype: Oʻahu , Kahana Bay ( 21.5573 N, - 157.8783 E, 15 m), 22.vii.2020, ♀, ex Xyleborus beetles from Schefflera actinophylla (deposited in UHIM). GoogleMaps
Allotype: Oʻahu, Kahana Bay ( 21.5573 N, - 157.8783 E, 15 m), 22.vii.2020, ♂, ex Xyleborus beetles from Schefflera actinophylla (UHIM).
Paratypes: Oʻahu, Kahana Bay ( 21.5573 N, - 157.8783 E, 15 m); 10.ix.2020; 11 ♀, 2 ♂; ex Xyleborus beetles from Schefflera actinophylla; ( 6 ♀, 2 ♂ UHIM; 5 ♀ BPBM) • Oʻahu, Kahana Bay ( 21.5573 N, - 157.8783 E, 15 m); 22.vii.2020; 18 ♀, 5 ♂; ex Xyleborus beetles from Schefflera actinophylla; ( 8 ♀, 1 ♂ UHIM; 5 ♀, 2 ♂ BPBM; 5 ♀, 2 ♂ NHMUK) • Oʻahu, Kahana Bay ( 21.5604 N, - 157.8765 E, 15 m); 31.i.2020; 3 ♀, 2 ♂; ex Xyleborus ferrugineus from Mangifera indica; (UHIM) • Hawaiʻi island, ʻĀhualoa; 30.x.2019; 1 ♀, 1 ♂; ex Xyleborus perforans from Macadamia integrifolia; (UHIM) • Hawaiʻi island, Keaʻau; 1.xi.2019; 4 ♀, 4 ♂; ex Xyleborus ferrugineus from Macadamia integrifolia; ( 2 ♀, 2 ♂ UHIM; 1 ♀, 1 ♂ BPBM; 1 ♀, 1 ♂ NHMUK) • 2 ♀, 1 ♂; same data as previous except ex Xyleborus affinis from Macadamia integrifolia; (BPBM) • 1 ♀, 1 ♂; same data as previous except ex Xyleborus perforans from Macadamia integrifolia; (NHMUK) • Oʻahu, Mānoa valley ( 21.3293 N, - 157.7972 E); 15.ix.2020; 3 ♀, 4 ♂; ex Xyleborus affinis from Cecropia obtusifolia; ( 1 ♀, 1 ♂ UHIM; 1 ♀, 2 ♂ BPBM; 1 ♀, 1 ♂ NHMUK).
Etymology. This wasp strolls along the surface of wood to search for its hosts. The species name is Hawaiian, meaning “to go out for a walk or to see the sights” and is to be treated as an indeclinable noun in apposition.
Biology
Known host range
Phymastichus holoholo sp. nov. has been reared from Xyleborus ferrugineus , X. perforans , and X. affinis adult beetles in octopus tree ( Schefflera actinophylla) in the Kahana Bay area of Oʻahu; the same three beetle species in macadamia ( Macadamia integrifolia) in the areas of ʻĀhualoa, Kapaʻau, and Keaʻau on the island of Hawaiʻi; from X. ferrugineus in mango ( Mangifera indica L.) branches in the Kahana Bay area of Oʻahu; and from X. affinis in trumpet tree ( Cecropia obtusifolia Bertol.) branches near the upper reaches of Mānoa valley on Oʻahu.
Emergence holes on the elytral declivity of the beetle, typical for this genus as observed in LaSalle (1995), Espinoza et al. (2009), and the present study, were found on two Hawaiian endemic Xyleborus lanaiensis Perkins beetles extracted from a fallen ‘ālaʻa ( Planchonella sandwicensis (A. Gray) Pierre) tree in the Waiʻanae mountains of Oʻahu ( Fig. 4i View FIGURE 4 ). We expect these beetles were parasitized by P. xylebori or P. holoholo , or perhaps another unknown Phymastichus species, but cannot know with certainty whether one or both of these species include any members of the Hawaiian endemic radiation of Xyleborus beetles in their host range. Hundreds of X. lanaiensis individuals were collected from the same tree and no emergence of parasitoids was observed, suggesting the percent parasitism of these beetles at this location in this tree was low. Another similar emergence hole was also found on an X. ferrugineus beetle in Acacia koa A.Gray on Waʻahila Ridge on Oʻahu, though again we do not know by which species it was parasitized.
We also observed two species of Scolytinae that this wasp may use under some circumstances but does not seem to prefer, at least in the environments we have observed it so far. An emergence hole typical of Phymastichus was found on a single Xylosandrus crassiusculus Motschulsky beetle inhabiting the octopus tree branches used in the calculation of percent parasitism (described later) ( Fig. 4j View FIGURE 4 ). Since all wasps emerging from beetles within this wood were P. holoholo we assume the beetle was parasitized by this species,. Though X. crassiusculus is a somewhat common inhabitant of the octopus tree wood in that area, and we have extracted a good number of individuals from those trees during this study (in excess of 100), this is the only individual we have seen that was clearly parasitized.
Though Euwallacea fornicatus ( sensu lato) beetles are also common inhabitants of the octopus tree wood from which we found this species emerging, we have not observed any individuals with a parasitoid emergence hole. Phymastichus holoholo individuals have also been observed to actively walk over E. fornicatus ( sensu lato) beetles naturally infesting octopus tree wood without showing any sign of interest, while showing substantial interest in and parasitism of Xyleborus beetles in the same stage of infestation. Despite this, two instances of parasitism have been observed in dissected E. fornicatus ( sensu lato) individuals: one parasitoid larva, confirmed by molecular analysis to be P. holoholo , and one seemingly developed adult that eclosed but had not yet emerged from the beetle, were found inside beetles extracted from naturally infested octopus tree wood and identified by DNA sequences as E. perbrevis (Schedl) and E. fornicatus (Eichhoff) , respectively (using the primers of Folmer et al. (1994); the CO1 gene matched with specimens on GenBank referenced in Smith et al. (2019)). Thus at least some P. holoholo individuals may under some circumstances take marginal interest in members of this species complex and are able to complete their development inside them, though such instances seem to be rare.
We also report a species of Scolytinae that we infer is likely not used as a host by this species. The coffee berry borer, Hypothenemus hampei (Ferrari), a very significant pest of coffee in almost all major growing areas outside its native range, also seems typically to be rejected as a host by both P. holoholo and P. xylebori . Hypothenemus hampei individuals (n = 24) were glued into holes both artificially drilled and naturally bored by X. affinis beetles in octopus tree wood, and these logs were placed in an area of Kahana Bay on Oʻahu where substantial parasitism of other bark beetles by P. holoholo was consistently observed. Upon subsequent rearing and dissection, none were found to have been parasitized. And though P. holoholo is known through other observations to readily attack its preferred hosts X. ferrugineus and X. affinis when they are placed together in small containers, laboratory experiments that exposed H. hampei adult females to P. holoholo and P. xylebori females in a Petri dish, following the methods of Yousuf et al. (2021) for host specificity testing of P. coffea , did not result in any successful parasitism of H. hampei (n = 10 for P. holoholo , n = 2 for P. xylebori ). In another laboratory test, 95 P. holoholo individuals were exposed to H. hampei, some drilling into coffee berries and others loose on the bottom of the test container. One P. holoholo individual was observed attempting to parasitize an H. hampei adult, though no progeny emerged from the beetle. Hypothenemus hampei is substantially smaller than the Xyleborus beetles which are confirmed hosts of P. holoholo and P. xylebori , and the wasp observed attempting parasitization was an atypically small female. Besides this one instance, no interest in H. hampei was observed.
The insects and plants recorded here as hosts of P. holoholo are not predicted to be a comprehensive list. More exploration will almost certainly bring records of additional plant species on which this species attacks Xyleborus beetles, and possibly of additional hosts. We do not know the native range of this wasp, though two of its hosts in Hawaiʻi, X. ferrugineus and X. affinis , are thought to be neotropical in origin, while the native range of X. perforans is not as clear ( Wood 2007).
Lifespan
Of 52 P. holoholo females and males placed in a cubic plexiglass box with sides approximately 30 cm in length and provided with honey as an energy source, 96% were dead between 48 and 72 hours after emergence, and 100% were dead between 72 and 96 hours after emergence. Their lifetime thus seems to be about 2 or 3 days under these conditions. A similar lifespan has been reported for adult P. coffea ( Espinoza et al. 2009) .
An approximation of the fecundity of P. holoholo
Mated adult P. holoholo females without a chance to have oviposited in any host were dissected and the number of eggs within their gasters were counted. Given their short lifespan, these wasps presumably would not be able to produce new eggs during their lifetimes and are likely proovigenic, so that the number of eggs counted likely represents an accurate estimate of their maximum fecundity. Ten individuals were dissected yielding a mean of 33.5 (SD = 7.0) eggs per individual. Two eggs are typically laid per host beetle (see the section: Development, emergence, and mating), which implies the mean maximum number of beetles a single female could parasitize is 16.75 (SD = 3.5).
Behavior
Searching and oviposition
Parasitism of Xyleborus beetles by P. holoholo was observed under field conditions periodically over approximately a one year period. Octopus tree wood bolts approximately 60 cm long and 5 to 8 cm in diameter were cut from living trees and suspended off conspecifics in an octopus tree forest in Kahana Bay on Oʻahu; after approximately 6 weeks they had become infested by a variety of wood associated insects including bark beetles. Phymastichus holoholo wasps were regularly observed searching and parasitizing Xyleborus beetles on these branches. Their behavior in this environment seems to follow this general pattern:
Phymastichus holoholo females fly near the surface of wood containing potential hosts, generally within about 20 cm in an undulating flight pattern, as if scanning the wood to determine its quality. They may then land on the surface of the wood, not necessarily in the direct vicinity of a potential host, and commence searching by walking over the wood surface. This stage of searching seems to be largely visually and tactically mediated, as opposed to using long range olfactory cues, based on the following observations: the wasps walk in a generally linear pattern with few abrupt changes in direction except when taking interest in a nearby feature on the surface of the wood or reaching an obstacle. While they often move in the direction of bark beetle holes that appear to be visible from their position (either the hole itself or a beetle frass pile coming out of it), they also seem not to be aware of ostensibly healthy host tunnels that are spatially close but separated from them by a visual barrier such as moss or a bump on the bark. They sometimes change direction to move towards visible protrusions in the wood, even when there is no beetle hole present. While these wasps do not seem to be guided to attractive beetle holes by long range olfactory cues when walking on the wood, these observations do not preclude the use of short range olfactory cues of a centimeter or so in host finding. More than fifty P. holoholo were observed searching on the wood surface. An example of this searching behavior is shown in Supplementary Video 1.
Upon discovery of a bark beetle hole, P. holoholo females were observed to either 1) take interest in it, visible to the human observer through slowing or arrestation of walking motion and antennation of the beetle itself or the entrance to the hole, or 2) show a lack of ostensible reaction by walking over, around, or past the hole without a clear change in pace. Xyleborus beetles are often observed to sit with the back of their elytra flush with the surface of the wood, presumably to protect their tunnels. A P. holoholo wasp that takes interest in a beetle in such a position will briefly antennate its elytra, and if it finds it acceptable quickly move onto the back of the beetle, raise its wings, insert the ovipositor into the declivity of the beetle between the elytra, and commence oviposition. The position it holds its wings during oviposition is similar to what has been recorded for P. coffea ( Espinoza et al. 2009) . P. holoholo has also been observed to enter a beetle tunnel to which it has taken interest if the beetle is not near the surface of the wood. It does this by backing down into the tunnel, digging its way through any frass or dust with its legs as it does so. This behavior of entering the host plant to find the beetle is in contrast with what is known for P. coffea , which is thought to only attack beetles exposed on the outside of the plant material while boring into coffee fruits ( Jaramillo et al. 2005).
Once oviposition commences, the beetle becomes agitated. It rotates itself in its tunnel and moves in and out, as if trying to shake off the ovipositing wasp. If the beetle was inside the tunnel when the wasp found it, the two are often observed coming back to the surface of the wood during this process as the beetle seems to attempt to clear the wasp out of the hole. This agitation does not often seem to much improve the beetle’s chances, as the wasp remains safely perched on the back end of the beetle, the beetle unable to abrade it against anything. In this effort, one beetle was observed falling onto the ground with the wasp attached to it, but in all other observations the beetle remained in its tunnel. This movement has also been observed to attract the attention of spiders and ants in the vicinity, though whether they might disturb the wasps is unknown.
Once oviposition has presumably concluded, the wasp removes its ovipositor and walks off the beetle. Searching by walking over the surface of the wood then often resumes, or the wasp flies off. Oviposition seems to last typically 2 or 3 minutes, though longer times up to 20 minutes have been observed. Oviposition times less than 30 seconds have also been observed, though it is unclear if these were successful ovipositions or aborted attempts. Full oviposition events were observed and described in detail 10 times, and numerous additional such interactions were also seen to occur. We know that P. holoholo will somewhat readily parasitize its hosts outside of wood tunnels in laboratory containers, but oviposition attempts on free-living beetles have not been observed in nature. See Supplementary Videos 2 and 3 for host acceptance and oviposition events. Male P. holoholo have not been observed on these bolts of wood.
Individual wasp females encountering each other on the surface of the wood seemed to show little interest in each other. Phymastichus holoholo individuals were also observed to come across other actively ovipositing P. holoholo while searching on wood. In most observed instances, they would briefly antennate the area and then move on to continue searching apparently without affecting the other wasp’s behavior, though on a few occasions the ovipositing wasp appeared disrupted and flew away.
Phymastichus holoholo seems potentially to be able to distinguish parasitized from unparasitized beetles. We have not confirmed this, but P. holoholo has been observed to walk directly over apparently alive and healthy host beetles without showing any sign of interest, and then go on to parasitize others of the same species. Ability to recognize hosts previously oviposited in by a conspecific and a preference for unparasitized hosts has also been observed for P. coffea ( Castillo et al. 2004) . Additionally, some frass piles from active beetle tunnels arrested the wasps while others were largely ignored, suggesting that P. holoholo might be able to distinguish host species using cues from the fresh frass around their tunnels.
Development, emergence, and mating
After hatching from their eggs, larval P. holoholo cleanly consume the insides of their host. Pupation was observed to typically occur with a larger female occupying the posterior of the beetle, its head facing the posterior end and its body extending to approximately the junction between the pro- and mesothorax, and a smaller male curled up facing transversely inside the prothorax ( Fig. 4 d,e View FIGURE 4 ). Similar location of pupae inside their host has been observed for P. coffea ( Espinoza et al. 2009) .
Emergence behavior was observed by collecting paralyzed (and thus potentially parasitized) X. ferrugineus and X. affinis beetles from octopus tree branches, gluing them close together in a Petri dish, and placing a video camera above to record emergence events.
Like P. coffea and P. xylebori , P. holoholo emerges from its host beetle by chewing a round exit hole in the middle of the elytral declivity ( Fig. 4 a,f,g,h View FIGURE 4 ). This process was observed to take between 61 and 256 minutes with a mean of 140 minutes (n = 7). After chewing a hole just large enough to emerge through, the female crawls out of the beetle and remains close to it. There is typically one male and one female wasp in each host, and the female emerges first, though sometimes only a single female would emerge. Out of 15 emergence events observed, 11 had one male and one female, and 4 had only a single female. A single male was never observed emerging without a female. Some time later, between 17 and 990 seconds with a mean of 330 seconds (n = 4), the smaller male crawled out of the hole and walked away from its host and the previously emerged female, as if exploring the area. This behavior is presumably to find a mate that is not its sister. In our setup, however, the only option was its sister, and after 8 to 246 seconds with a mean of 70 seconds (n = 4), the male returned to its emergence location, where the sister was still waiting. The pair did a courtship routine followed by mating. After this, both the male and female flew off. If no male emerged, the female was observed to wait 36 to 45 minutes with a mean of 41 minutes (n = 3) next to its emergence site before flying off. This mating behavior contrasts with that of P. coffea , which is thought to have sibling mating inside its host prior to emergence ( Espinoza et al. 2009). It is also not common among parasitoids that the female waits for the male to emerge for mating, but maybe understandable for this species given its short lifespan and the consequent necessity to mate quickly to oviposit in a new host. See Supplementary Video 4 for an example of emergence and mating.
Percent parasitism in octopus tree wood in Kahana Bay, Oʻahu
Six octopus tree wood bolts 5 to 8 cm in diameter and 60 cm long were cut fresh and suspended from a metal hanger approximately 2 m off the ground in an octopus tree forest in Kahana Bay, Oʻahu ( 21.5573 N, - 157.8783 E, 15 m). After 6 weeks, on 22 July 2020, the wood was taken down and dissected completely. Every beetle tunnel was followed to its end, first by peeling the bark and thoroughly searching it, and then by splitting the xylem apart with a hammer, chisels, and pliers. All Xyleborus adult beetles were removed and placed in screened boxes for parasitoid emergence. After 5 weeks, beetles with wasp emergence holes were counted and emerged wasps were collected. Emergence conditions were likely less than ideal, so all remaining beetles were dissected to find Phymastichus immatures or adults inside that had not fully developed or emerged. All Phymastichus emerging from these bolts were P. holoholo , so immatures inside the beetles were assumed to also be P. holoholo .
From past observations cutting and suspending similar octopus tree logs from trees in the same area, bark beetles typically begin to enter the wood approximately 4 weeks after it is cut: a second generation of adults could thus not have been produced in the 6 weeks the wood was exposed to the environment before it was dissected. The calculated percent parasitism of beetles collected from the wood can thus be considered an accurate measure of the percent of beetles colonizing the wood that were parasitized by P. holoholo .
In total, 53 of 86 (62%) X. ferrugineus adult beetles collected from the wood were parasitized. 124 of 152 (82%) X. affinis adult beetles were parasitized. A single X. perforans beetle was present and it was parasitized. Thus the total percent parasitism for all Xyleborus beetles was 74%.
| UHIM |
UHIM |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |