Phyxioschema suthepium Raven & Schwendinger, 1989
|
publication ID |
https://doi.org/10.5281/zenodo.188258 |
|
DOI |
https://doi.org/10.5281/zenodo.6214224 |
|
persistent identifier |
https://treatment.plazi.org/id/03A9B15A-FFA3-7E70-FF06-B6C0FA5DFD8B |
|
treatment provided by |
Plazi |
|
scientific name |
Phyxioschema suthepium Raven & Schwendinger, 1989 |
| status |
|
Phyxioschema suthepium Raven & Schwendinger, 1989
Figures 2–3 View FIGURE 2 A – D View FIGURE 3 A – L , 22B–C View FIGURE 22 A – I
Phyxioschema suthepia Raven & Schwendinger, 1989: 56 –59, figs 1–10 (description of male and female). Phyxioschema suthepium: Platnick 1993: 91 (mandatory change of species name ending); Raven & Schwendinger 1995: 639 –640, fig. 11A–F (emendation of diagnosis).
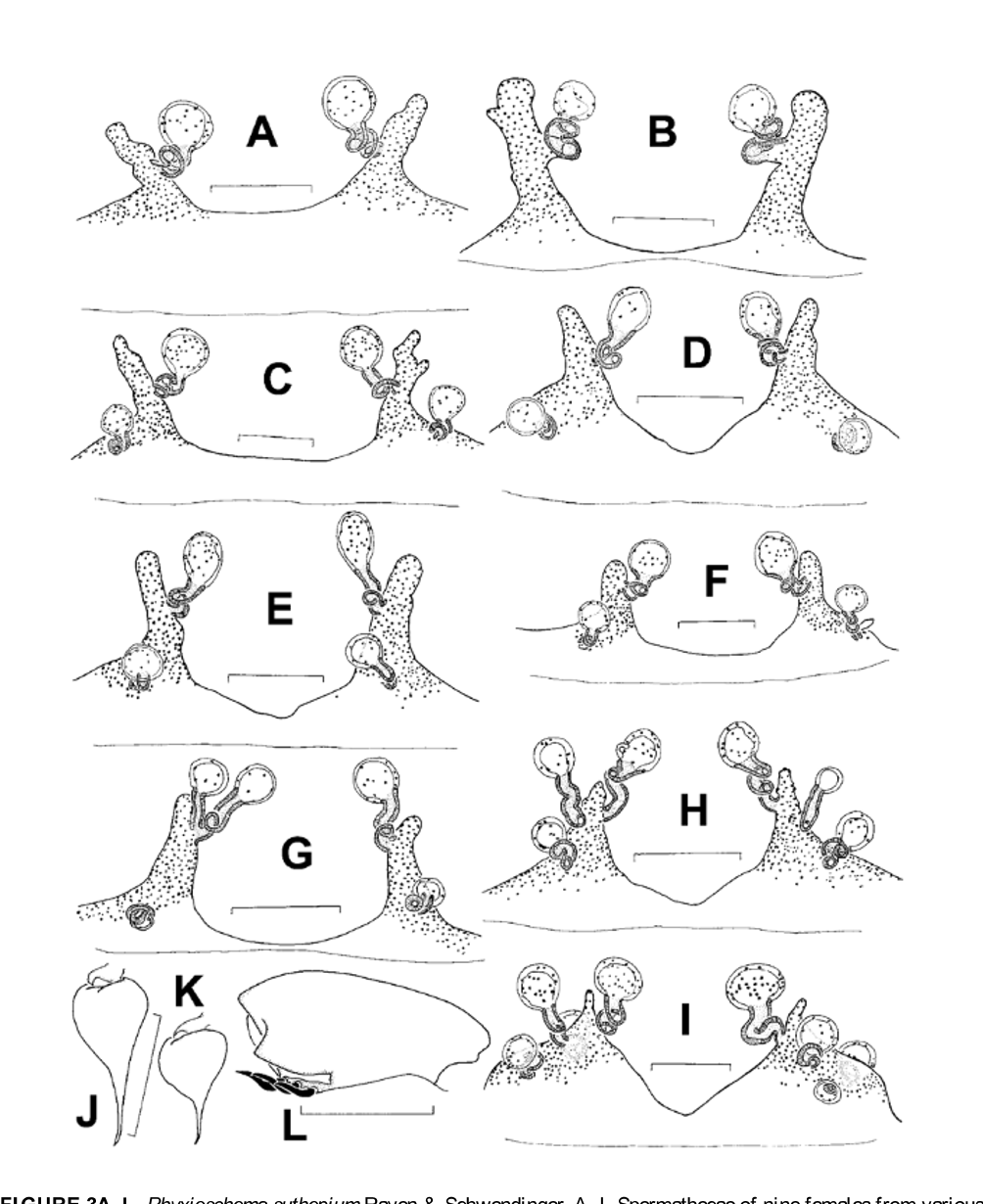
Emended diagnosis: This is the smallest species in the genus. It differs from all known congeners by mostly aspinose leg tarsi in both sexes (usually only one retrolateral spine present on tarsus IV). Males with palpal tibia ( Fig. 2C View FIGURE 2 A – D ) relatively shorter and stouter than in other Phyxioschema species; all leg tarsi integral (not pseudosegmented); tibia I (cylindrical as in all known species from Thailand) without retroventral and retrolateral spines, a condition otherwise only found in P. s a y a m e n s e sp. n. ( Fig. 13G View FIGURE 13 A – L ); patella I with a row of two to (mostly) three short, sigmoid spines retroventrally and with a triangular, scale-like projection on its retrolateral margin ( Fig. 3L View FIGURE 3 A – L ; not mentioned in the original description but indistinctly visible in one of the illustrations in there, see Raven & Schwendinger 1989: fig. 1; this structure presumably further supports interlocking of legs I and II during copulation); retrodorsal band of hooked spinules on femur I quite short and wide ( Fig. 2A View FIGURE 2 A – D ); proventral band of such spinules on femur II long and narrow ( Fig. 2B View FIGURE 2 A – D ); prolateral band of elongated spinules on tibia II strongly curved and distinctly inclined from longitudinal axis of tibia ( Fig. 2D View FIGURE 2 A – D ). Females with strongly convoluted stalks of the median and secondary receptacles and with basally wide (without a constriction), unsclerotised lateral receptacles ( Fig. 3A–I View FIGURE 3 A – L ).
Relationships: Phyxioschema suthepium appears most closely related to P. erawan sp. n. These two species are quite small, possess metatarsal preening combs, have unsclerotised lateral receptacular bases, live on the ground (cf. the other Thai species on limestone) and occur in geographical proximity to each other (see Fig. 1 View FIGURE 1 , localities 2–13 and 1, 14, respectively).
Variation: Intraspecific and within-population variation in the shape of the female genitalia examined is quite large ( Fig. 3A–I View FIGURE 3 A – L ). The number of receptacles per spermathecal trunk varies from two ( Fig. 3A View FIGURE 3 A – L ) to five ( Fig. 3I View FIGURE 3 A – L ). Females from southern populations generally have more secondary receptacles than those from northern populations; those from Chiang Mai Province usually have none at all. The spermathecal trunks are mostly quite narrow and more or less distinctly inclined from each other (most distinctly so in females from northern populations; Fig. 3A–E View FIGURE 3 A – L ); only in one female from Ko Samet ( Fig. 3I View FIGURE 3 A – L ) are they wide. However, a second female examined from Ko Samet possesses quite typical genitalia ( Fig. 3H View FIGURE 3 A – L ). All females from Ban On Luai examined (n=4) have the base of the lateral receptacle constricted to a neck (as shown in Fig. 7A–B View FIGURE 7 A – H for P. erawan sp. n.); they also lack secondary receptacles. Variation in size and shape of palpal bulbs is high in males from the same population at Sai Yok ( Fig. 3J–K View FIGURE 3 A – L ; n=4, only extremes illustrated). See also the variation in the shape of the tibia II coupling spur and in the number of the corresponding megaspines illustrated by Raven & Schwendinger (1995: fig. 11A–D).
On metatarsus I, preening combs (composed of two or three stiff setae) are present or absent (often both conditions present on one specimen); metatarsi II–IV always have preening combs. Small males (e.g., from Si Racha and Khao Phanom Sawai) lack spines on tarsus IV, one larger male has a single retrolateral spine also on tarsus III (only on one side), one female has two retrolateral spines on tarsus IV (only on one side).
Remarks: Raven & Schwendinger (1995: 639, fig. 11A–D) corrected a false distinction given in Raven & Schwendinger (1989: 55–56): " P. raddei possessing two megaspines on the tibial spur of males and P. suthepium three megaspines". P. suthepium males normally also possess two megaspines (as do males of all other known congeners) and only exceptionally three (as unfortunately is the case in the holotype). A second distinction is also incorrect: " P. suthepium without foveal setae". All known Phyxioschema species possess foveal setae. In some males of P. suthepium , these setae are slightly less pronounced (often also situated closer to or even within the fovea) than in P. raddei females examined (including the holotype, in which the foveal setae are just anterior to the fovea), but in P. suthepium females they are always clearly visible.
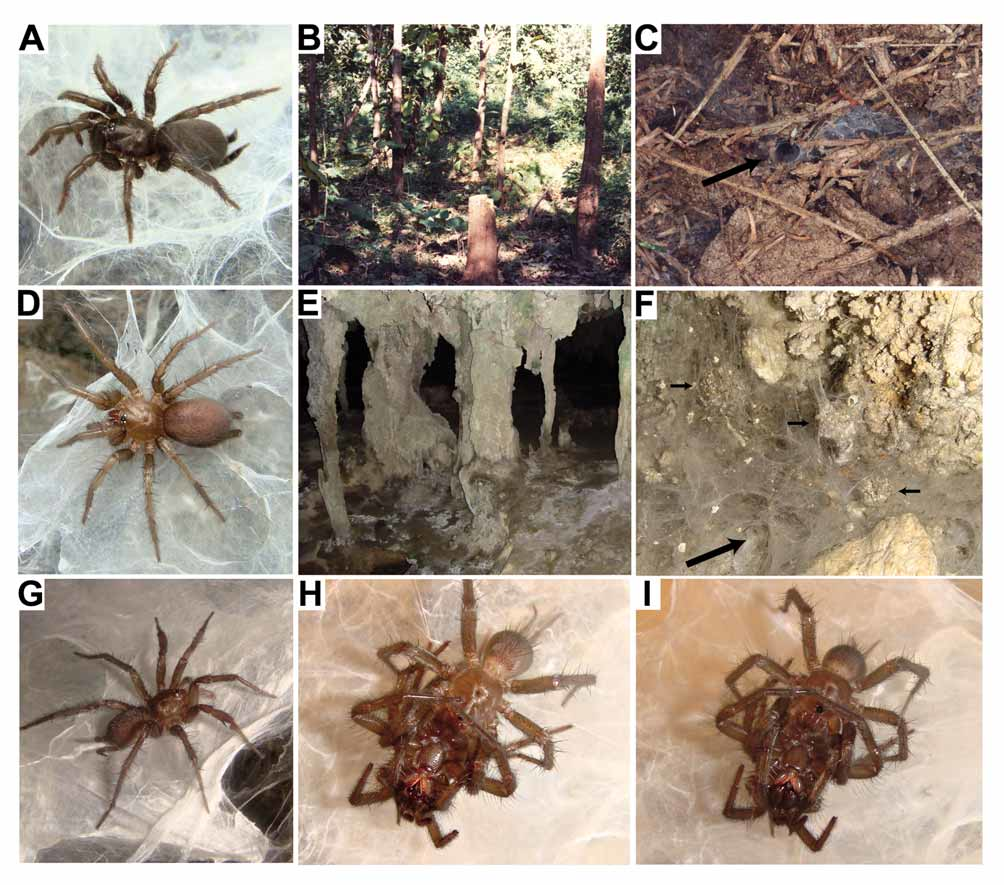
Distribution and habitat: This species is widely distributed and locally common in the lowlands of northern, western, central and north-eastern Thailand. The highest known locality is Doi (= Mount) Khuntan, at 620 m. Phyxioschema suthepium was described from Chiang Mai ( type locality) and other localities in the north ( Raven & Schwendinger 1989: 56) and later also reported from areas in central Thailand ( Raven & Schwendinger 1995: fig. 10). New material is available from the west and northeast of the kingdom. The currently known localities (with sex of available specimens and name of province in parentheses) are: Pha Yao (females; Pha Yao Province), Doi Suthep (males, females), Ban Mae Hia (males, females), Chiang Mai (males, females), Ban On Luai near Sankamphaeng (females; Chiang Mai Province), Doi Khuntan (females; Lamphun Province), Mae Sariang (females), Sop Moei (females; Mae Hong Son Province), Lam Nam Nan (males, females; Uttaradit Province), Khlong Lan National Park (females; Kamphaeng Phet Province), Khao No (females; Nakhon Sawan Province), Khao Phanom Sawai (males, females; Surin Province), Ko Samet (females; Rayong Province), Si Racha (males, females; Chon Buri Province), Sai Yok National Park (males, females; Kanchanaburi Province); see Fig. 1 View FIGURE 1 , localities 2–13. All spiders were found in small webs in holes and cracks in the soil, and under stones, dead wood and leaves lying on the ground. They are more prevalent in dry habitats such as roadsides, cut paths (i.e., the earthbanks on either side of paths) and open forests (secondary forests, teak plantations, orchards) and have not been found in evergreen forests with a closed canopy. At Ban On Luai (ca 25 km east of Chiang Mai), these spiders were found on the slopes of an isolated limestone hill, a habitat otherwise typical for P. erawan sp. n.
Population dynamics: Fluctuations in the size of a population living in a 19 m 2 area of teak plantation ( Fig. 22B View FIGURE 22 A – I ) at Ban Mae Hia near Chiang Mai were monitored in 1986 by counting webs ( Fig. 22C View FIGURE 22 A – I ) at monthly intervals. Density was high (40 webs) in January, after the end of the rainy season and into the cool-dry season. In February, all webs in that plot were destroyed by a forest fire. During the hot-dry season recolonisation was very slow (one web in March, three in April) but increased drastically with the onset of the rainy season (35 webs in May). The counts decreased to 28 and 22 webs in June and July, respectively, and reached a high of 56 in August. P. suthepium is thus a pioneer species that can rapidly colonise new or degraded habitats in fairly large numbers. Forest degradation caused by human activities has probably paved the way for the unusually wide distribution range of this species in seasonally dry areas of Thailand.
Phenology ( Table 3): In 1985–1988, two periods of reproductive activity per year were observed by means of pitfall trapping, hand collecting and captive rearing in Chiang Mai. In one, mature males were present from early December to late February; egg sacs were constructed in February to May, and spiderlings hatched about three weeks after oviposition. In the other, mature males were present from early May to late July, eggs were laid in June and July and hatched about two weeks later ( Raven & Schwendinger 1989: 59). Individual males reproduced only during one of these two periods each year and then died within a few weeks.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Phyxioschema suthepium Raven & Schwendinger, 1989
| Schwendinger, Peter J. 2009 |
Phyxioschema suthepia
| Raven 1995: 639 |
| Platnick 1993: 91 |
| Raven 1989: 56 |