Obama apiguara Oliveira, Almeida & Carbayo, 2020
|
publication ID |
https://doi.org/10.5852/ejt.2020.705 |
|
publication LSID |
lsid:zoobank.org:pub:B05B3C54-31C8-42C4-940F-63354D573678 |
|
DOI |
https://doi.org/10.5281/zenodo.4328412 |
|
persistent identifier |
https://treatment.plazi.org/id/CBCCCD96-212C-4958-AE86-7CB5AE6E1653 |
|
taxon LSID |
lsid:zoobank.org:act:CBCCCD96-212C-4958-AE86-7CB5AE6E1653 |
|
treatment provided by |
Valdenar |
|
scientific name |
Obama apiguara Oliveira, Almeida & Carbayo |
| status |
sp. nov. |
Obama apiguara Oliveira, Almeida & Carbayo sp. nov.
urn:lsid:zoobank.org:act:
Figs 1–5 View Fig View Fig View Fig View Fig View Fig
Diagnosis
A species of Obama with a dark ivory dorsum, dark tips and numerous small longitudinal black striae and a glandular fossae opening through the epidermic epithelium. The subintestinal parenchymal muscle is intermingled with the nerve plexus. The penis papilla is provided with a small intra-papillar, fingerlike papilla. A common glandular ovovitelline duct is absent. The female genital duct projects from the mid-dorsal section of the female atrium.
Etymology
The name, ' apiguara' ( apyguara) is a Tupi (indigenous Brazilian language) word, meaning ' nasal fossae ' ( Bueno 1998). It refers to the glandular fossae opening through the epidermis.
Material examined
Holotype
BRAZIL • 1 adult; State of Santa Catarina, Três Barras, Parque Nacional de São Joaquim ; 28.2356° S, 49.4988° W; 6 Sep. 2017; F. Carbayo et al. leg.; sagittal sections of copulatory apparatus on 54 slides, transverse sections of cephalic region on 26 slides, horizontal sections of portion containing ovaries on 11 slides, sagittal sections of posterior portion containing ovaries on 23 slides, sagittal sections of pharynx region on 32 slides, transverse sections of pre-pharyngeal region on 11 slides; field number F7378; MZUSP PL2187 . GoogleMaps
Type locality
Três Barras (Parque Nacional de São Joaquim), state of Santa Catarina, Brazil.
Description
MEASUREMENTS. The holotype, preserved, is 56 mm long and 8 mm wide.
BODY. Broad and flattened dorsoventrally. The body margins are nearly parallel, except the anterior 25%, and the posterior 17% of the body, which are pointed and obtuse, respectively. The dorsum is slightly convex, the ventral side flat. The color of the dorsum of the living specimen is dark ivory, pigmented and with numerous small longitudinal black striae formed by small spots. The anterior and posterior extremities, each 10–15% of body length, exhibit a black pigment. This pigment almost covers the striae ( Fig. 1A View Fig ). The ventral side is pastel-yellow in color, whitish in the region of the pharynx and the copulatory apparatus, and brown in both extremities (17% of the length of the body) ( Fig. 1B View Fig ).
EYES. Single-lobed and without halos, measuring about 50 μm in diameter. They are absent at the very anterior apex of the body ( Fig. 2A View Fig ) and are initially uniserial. Behind 4 mm (7% of body length from anterior tip), they are arranged in 2–3 marginal rows, which reach, on each side, approximately 0.7 mm of the total body width (1.25% of the body width). The eye distribution reaches the region of the gonopore, behind it becoming scarcer until the posterior tip of the body. The sensory pits are about 45 μm deep, and are distributed in a single ventro-marginal row. They contour the anterior extremity of the body and are distributed along the body until 14 mm from anterior extremity (25% of body length).
CREEPING SOLE. Occupies approximately 96% of the body width. The positions of the mouth and the gonopore relative to the anterior tip are about 70% and 85% of the body length, respectively. In the prepharyngeal region ( Fig. 2B View Fig ), the dorsal epithelium is crossed by necks of rabditogen cells, especially on the sides of the body; it is also crossed by necks of cells producing fine ( 1.2 µm) cyanophil granules and by necks of scarce cells producing xanthophil and erythrophil granules, respectively. The ventral epithelium is traversed by an abundant number of necks of cells producing cyanophil granules and a low number of necks of cells producing either xanthophil or erythrophil fine granules. In the body margins, the density of gland cells slightly increases giving rise to a poorly delimited glandular margin ( Fig. 2B View Fig ).
ANTERIOR 45% OF THE BODY. Presents a structures herein called glandular fossae. These glandular fossae are distributed irregularly in a simple or double row along the dorsal and body margins ( Fig. 3 View Fig ). A few fossae are also found in the ventral body margins ( Fig. 3E View Fig ). A glandular fossa consists of a 30–70 µm deep invagination, mostly simple ( Fig. 3C View Fig ), sometimes bifurcate ( Fig. 3D View Fig ). The invaginations are lined with a 5–15 µm high epithelium, that is lower than surrounding body epithelium ( 25 µm high). Cells of the lining epithelium are strongly erythrophil. The lumen of the fossae seem to contain red-pinkish threads and fine erythrophil granules. Necks of glands producing xanthophil secretions are located in surrounding parenchyma of the fossae, but they apparently do not discharge their secretion into the fossae but through surrounding epidermal cells.
CUTANEOUS MUSCULATURE. Organized in three layers: a subepithelial layer of circular fibers, followed by a layer of double diagonal fibers and a longitudinal one with bundles composed of 32–96 fibers each. The thickness of the cutaneous musculature is 10% of the body height in the pre-pharyngeal region. The parenchymal musculature is composed of a dorsal layer of diagonal fibers, followed by a supraintestinal layer of transverse fibers and a subintestinal layer of transverse fibers extending below the nerve plate, thus intermingled with it. The main nervous system presents the shape of a plate ( Fig. 2C View Fig ).
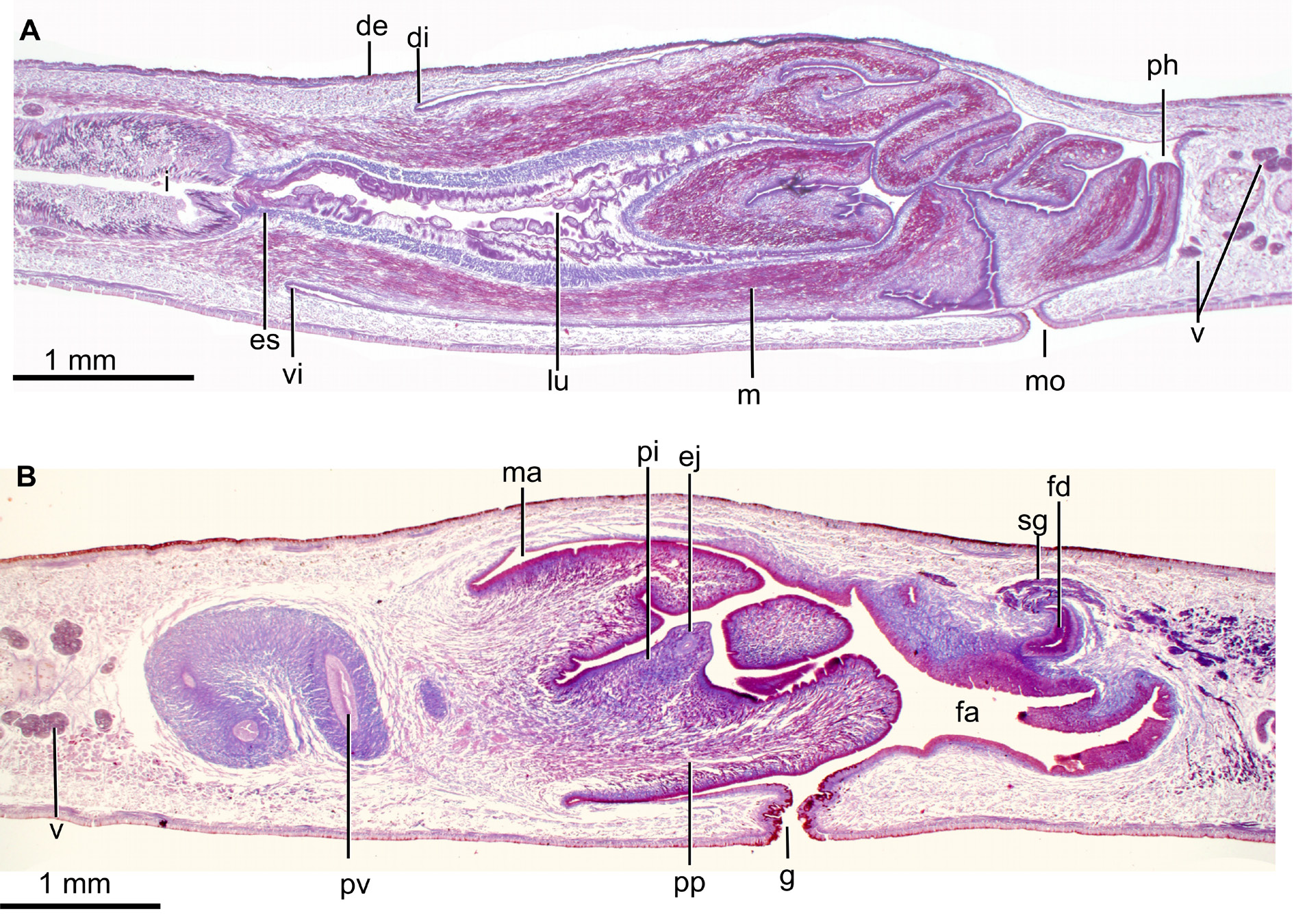
MOUTH. Opens at the end of the second third of the pharyngeal pouch. The pharynx is cylindrical ( Fig. 4A View Fig ), with the dorsal insertion posteriorly shifted. It occupies 77% of the length of the pharyngeal pouch. An esophagus is present; under its lining epithelium, there is a one-fiber-thick muscle layer followed by a layer ( 50 µm) of circular fibers interspersed with longitudinal fibers. The pharynx is lined with a cuboidal, ciliated epithelium. The pharyngeal epithelium is crossed by necks of three types of gland cells producing erythrophil, cyanophil and xanthophil granules, respectively. The outer pharyngeal epithelium is lined with a longitudinal muscle ( 5 µm), followed by a circular muscle ( 5 µm). The inner pharyngeal epithelium is lined with a a layer ( 100 µm) of circular fibers interspersed with longitudinal fibers, followed by a one-fiber-thick layer of longitudinal fibers.
TESTES. Dorsal, with a round shape, the largest being about 300 µm in diameter. They are arranged in 3–4 rows on each side of the body, starting 13 mm behind the anterior extremity of the body and reaching 20.5 mm, which corresponds to 23% and 37% of the body length, respectively.
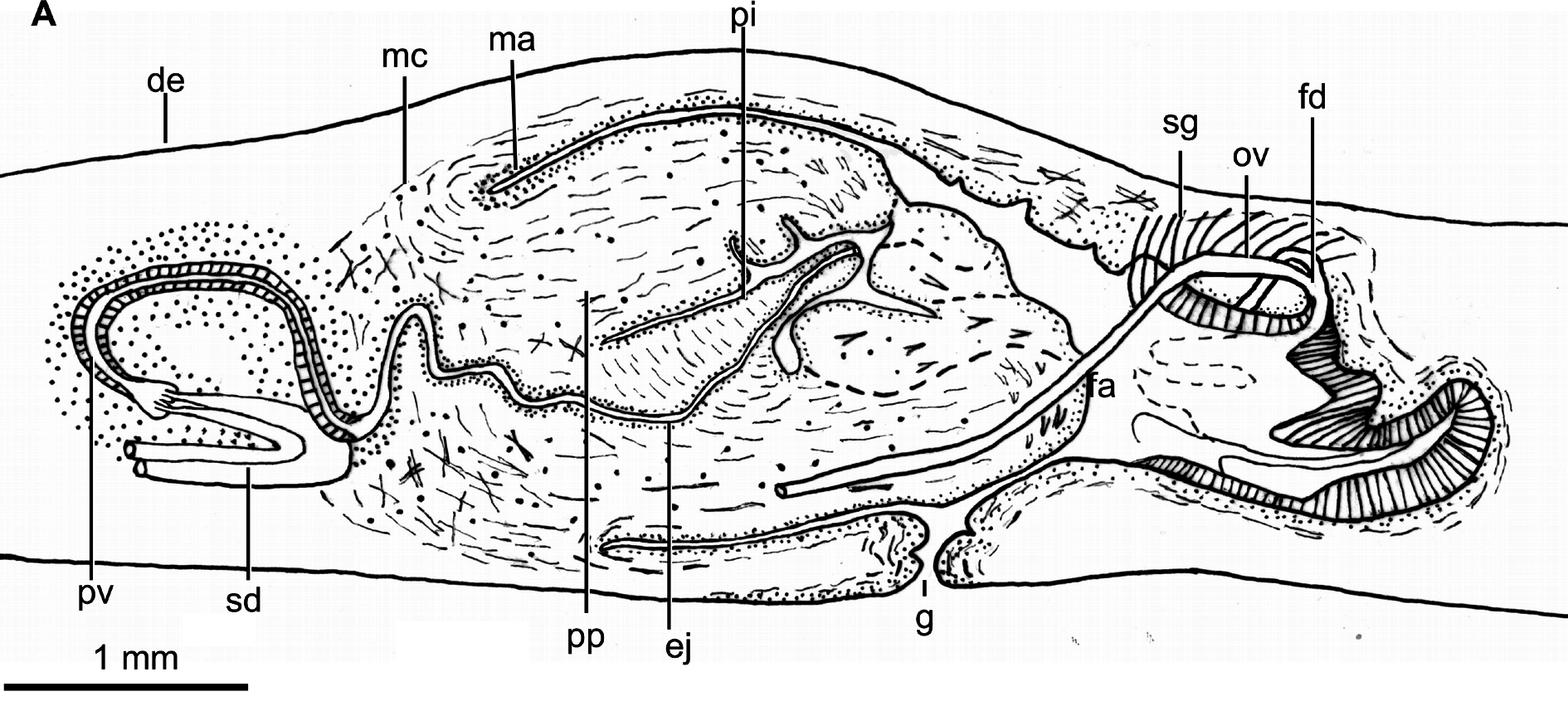
EFFERENT DUCTS. Bend upwards and forwards before ending in the proximal portion of the prostatic vesicle. The prostatic vesicle is extrabulbar, elongate and exhibits an inverted-U shape in lateral view. The proximal portion of this vesicle is forked. The vesicle enters the ventro-anterior section of the penis bulb to communicate with the ejaculatory duct. The prostatic vesicle is lined with a columnar, ciliated epithelium which is crossed by necks of cells producing erythrophil granules. This epithelium is surrounded by a circular muscle ( 160 µm thick), the ectal fibers of which are decussate.
EJACULATORY DUCT. Meanders in the first half of its length and becomes straight in the second half. This duct crosses the center of the intrapenial papilla, and its lining epithelium is columnar ( 25 µm tall) and ciliated. This epithelium is crossed by necks of cells producing erythrophil granules in its first half; in its second half, it is pierced by necks of cells producing cyanophil granules. The epithelium is surrounded by a circular muscle ( 30 µm thick).
PENIS PAPILLA. Larger than the male atrium and cylindrical, with its dorsal insertion slightly anterior to its ventral. It is provided with a finger-like intra-penial papilla with 600 µm in length, which is oriented postero-dorsally ( Figs 4B View Fig , 5 View Fig ). The epithelium of the intra-penial papilla is 5 µm high, and crossed by necks of cells producing erythrophil and cyanophil granules, respectively. This epithelium is underlain by a 2 µm thick longitudinal muscle, followed by a circular muscle ( 6 µm thick). This intra-penial papilla has radial muscle fibers and is provided with a sort of penis bulb constituted by crossed fibers. The large penis papilla is lined with a cuboidal-to-columnar epithelium, which is pierced by necks of abundant cells producing erythrophil granules. This epithelium is underlain by a circular muscle ( 15 µm thick), followed by a longitudinal muscle ( 20–30 µm thick), both layers being intermixed close to the insertions of the penis papilla.
MALE ATRIUM. Large and not folded, lined with a squamous-to-cuboidal epithelium, which is crossed by necks of cells producing erythrophil granules and a low number of necks of cells producing xanthophil granules. This epithelium is underlain by a circular muscle ( 17.5 µm thick), which in some areas presents intermingled longitudinal fibers.
OVARIES ( Fig. 2C View Fig ). Ovoid, 550 µm in length and 300 µm in width, approximately. They are situated at a distance from the anterior extremity of the body, equivalent to 27% of the body length. The ovovitelline ducts emerge from the dorso-lateral region of the ovaries and subsequently run above the nerve plate, ventrally to the efferent ducts. At the level of the gonopore, the ovovitelline ducts bend dorsally to join the female genital duct, which is a projection of the dorso-medial wall of this atrium ( Fig. 5 View Fig ). There is no common glandular ovovitelline duct. The distal portion of the ovovitelline ducts receives secretions from the shell glands. The female genital duct is lined with a 75 µm high, non ciliated epithelium. This epithelium is crossed by necks of two types of cells producing erythrophil and cyanophil granules, respectively.
FEMALE ATRIUM. Large and as long as the male atrium. The anterior portion of the atrium does not narrow and continues with the male atrium ( Figs 4B View Fig , 5 View Fig ). The female atrium exhibits 2–3 lateral folds that partially occupy its lumen. It is lined with a non-ciliated epithelium, with a stratified appearance in its posterior region. Towards the anterior portion of the atrium, this epithelium passes progressively to cuboidal ( 30 µm high, ventrally) or columnar ( 75 µm high, dorsally), and is crossed by necks of cells producing erythrophil granules and by a low number of necks of cells producing coarse cyanophil granules. The posteriormost section of the female atrium is pierced by cells producing a xanthophil amorphous secretion. The epithelium of the atrium is underlain by a longitudinal muscle ( 8 µm thick), followed by a circular muscle; fibers of both muscles are intermingled in some parts.
Remarks
This new species best matches Obama , since it presents the diagnostic features of this genus, with the exception of the eyes, which are only monolobate, the small intra-papillar finger-like penis papilla, and the female genital duct projecting from the dorsal side of the female atrium.
Among the 39 members of Obama , only four species also display a similar dorsal color pattern consisting of longitudinal dark small dots on a light ground color, namely O. allandra Marques et al., 2018 , O. nungara Carbayo et al., 2015 , O. maculipunctata Rossi et al., 2015 and O. marmorata (Schultze & M̹ller, 1857). Among them the striae in O. marmorata and O. nungara only form irregular longitudinal stripes as in the new species. However, in O. nungara , the irregular stripes are wider and the ground color is darker. Obama marmorata is very similar to the new species. Differences are related to the stripes, which are more evident in O. marmorata , and to the darker anterior and posterior body tips, restricted to a short body portion in O. marmorata .
Among the species of Pseudogeoplana , only Ps. blanchardi (Graff, 1899) and Ps. doederleine (Shirch, 1929) somewhat resemble our species in the marbled aspect of the dorsum. However, in Ps. blanchardi , from Venezuela, the dark spots are rounded (vs striated in O. apiguara sp. nov.); in Ps. doederleine , from Rio Doce (Rio de Janeiro), the spots are absent in a midband which is ornamented with four brownish thin longitudinal stripes (vs absent in Obama apiguara sp. nov.).
Regarding internal morphology, the copulatory apparatus of Obama apiguara sp. nov. resembles that of O. applanata (Graff, 1899) and O. carrierei (sensu Marcus, 1951) in that the latter species present a structure similar to an intra-penial papilla as in the new species (see Marcus 1951: fig. 169; Froehlich 1956: fig. 2). However, O. applanata and O. carreirei differ from the new species in the following aspects: they a) present the dorsal insertion of the penis papilla shifted posteriorly (vs anteriorly in the new species); b) possess a common glandular ovovitelline duct (vs absent in Obama apiguara sp. nov.); and c) their female genital duct projects from the posterior ( O. carreirei ) or postero-dorsal aspect ( O. applanata ) of the female atrium (vs dorsal). In addition, to the best of our knowledge, the new species is the only land planarian having glandular fossae opening through epidermis.
Distribution
Only known from the type locality.
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Continenticola |
|
Family |
|
|
Genus |