Adelophrnne nordestina, 2021
|
publication ID |
https://doi.org/10.1655/herpetologica-d-20-00022.1 |
|
DOI |
https://doi.org/10.5281/zenodo.7718665 |
|
persistent identifier |
https://treatment.plazi.org/id/03AB0E3F-FFA5-B14B-E9AE-8CD196EFFBD3 |
|
treatment provided by |
Felipe |
|
scientific name |
Adelophrnne nordestina |
| status |
sp. nov. |
Adelophrŋne nordestina sp. nov.
( Table 2 View TABLE ; Figs. 1–3 View FIG View FIG View FIG , 5 View FIG )
Adelophrŋne baturitensis Loebmann et al. (2011) :75. Adelophrŋne sp.1 Fouquet et al. (2012):550.
Adelophrŋne sp. Mesquita et al. (2018):459.
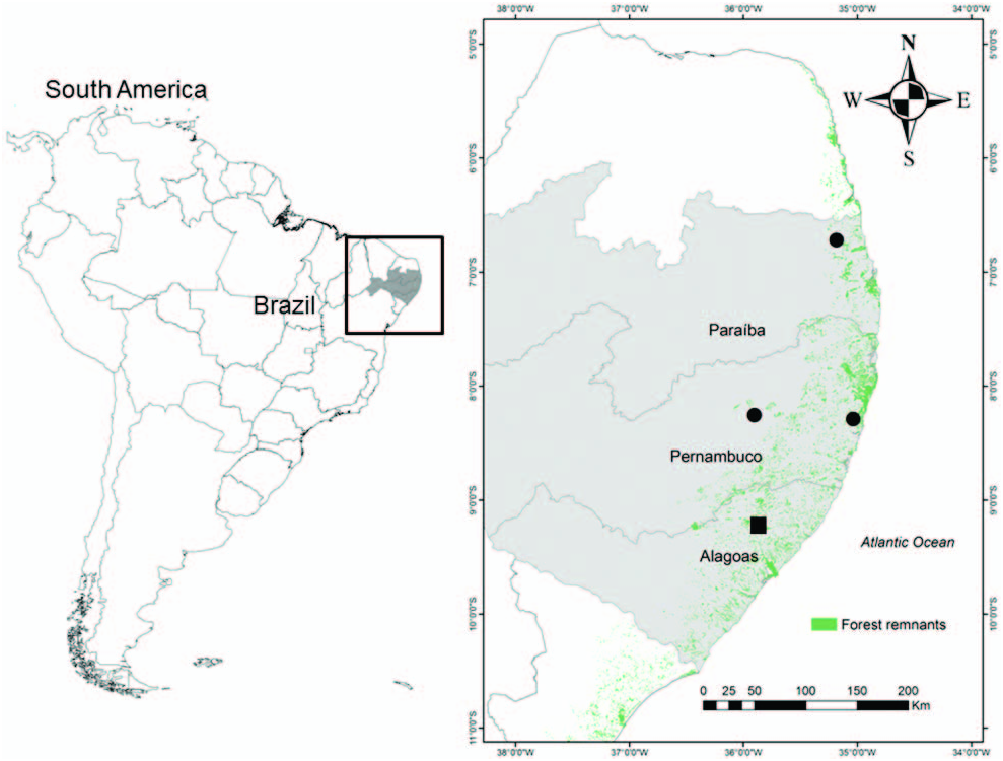
Holotype. — Adult male ( MUFAL 11771 ; Fig. 1 View FIG ) collected by B.S. Lisboa on 29 September 2013 in a forested area at the Mata da Bananeira , Estação Ecológica de Murici (09°12 ′ 45.72 ′′ S, 35°52 ′ 21.75 ′′ W; 597 m a.s.l.), municipality of Murici ( ESEC Murici), Alagoas State, Brazil. GoogleMaps
Paratypes. —Three adult females ( CHP UFRPE 3005 , 3007 , 3009 ) and two adult males ( CHP UFRPE 3006 , 3008 ) collected by C.C.M. Moura and E.B.F. Lisboa from the municipality of Cabo de Santo Agostinho (08°17 ′ S, 35°2 ′ W; 29 m a.s.l.), Pernambuco State, Brazil GoogleMaps ; six adult males ( MUFAL 10850 , 11064 , 11767–11770 ) and one adult female ( MUFAL 10851 ) from the holotype locality collected by B.S. Lisboa ; and one adult male ( ZUFMS-AMP13680 ), one adult female ( ZUFMS-AMP13679 ), and two juveniles ( ZUFMS-AMP 1367 View Materials and AMP1368 View Materials ) collected by L.O. Drummond, M. Wachlevski, M. Almeida-Gomes, M. Almeida-Santos, and P. Nogueira-Costa from the Reserva Biológica Guaribas ( REBIO Guaribas; 06°43 ′ S, 35°10 ′ W; 118 m a.s.l.) in the municipality of Mamanguape , Paraíba State, Brazil GoogleMaps .
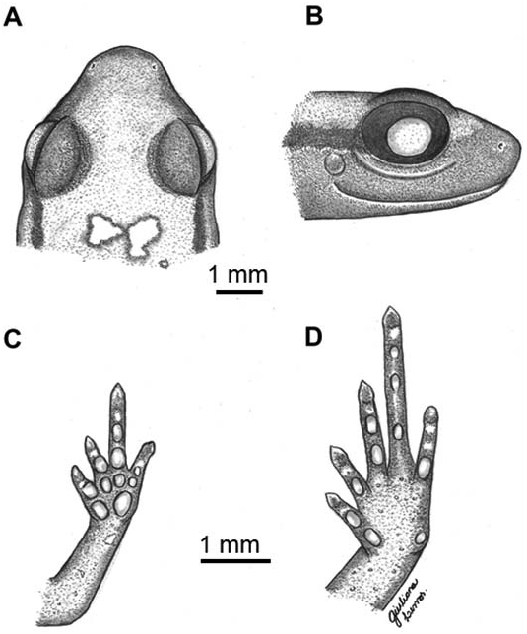
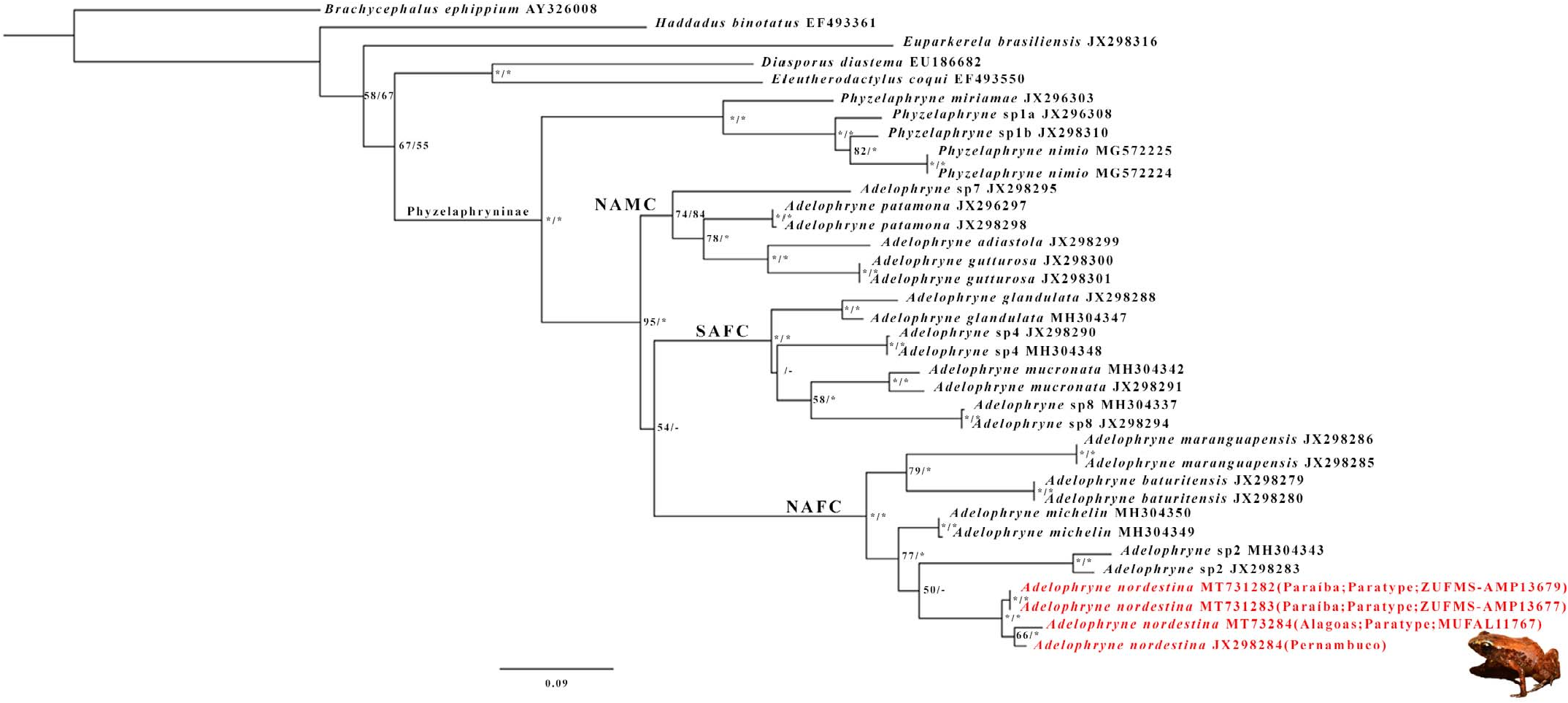
Diagnosis. —The new species is included in the subfamily Phyzelaphryninae due to the combination of the presence of apically pointed digits, terminal digits either barely or not expanded ( Hedges et al. 2008), and SVL not exceeding 23.0 mm ( MacCulloch et al. 2008). The new species can be distinguished from species in the genus Phŋzelaphrŋne by not having subarticular tubercles under the fingers, which are usually present in Phŋzelaphrŋne ( Hoogmoed and Lescure 1984; Hedges et al. 2008). The generic assignment of Adelophrŋne nordestina sp. nov. is based on the combination of possession of a head narrower than the body; small size; cranial crests absent; with subdigital pads and mucronate tip on the fingers I, II, III, and IV; toes I, II, III, IV, and V with subarticular tubercles, discs, and mucronate tips; terminal phalanges of toes and fingers Tshaped ( Hoogmoed and Lescure 1984); and its molecular phylogenetic position ( Fig. 4 View FIG ).
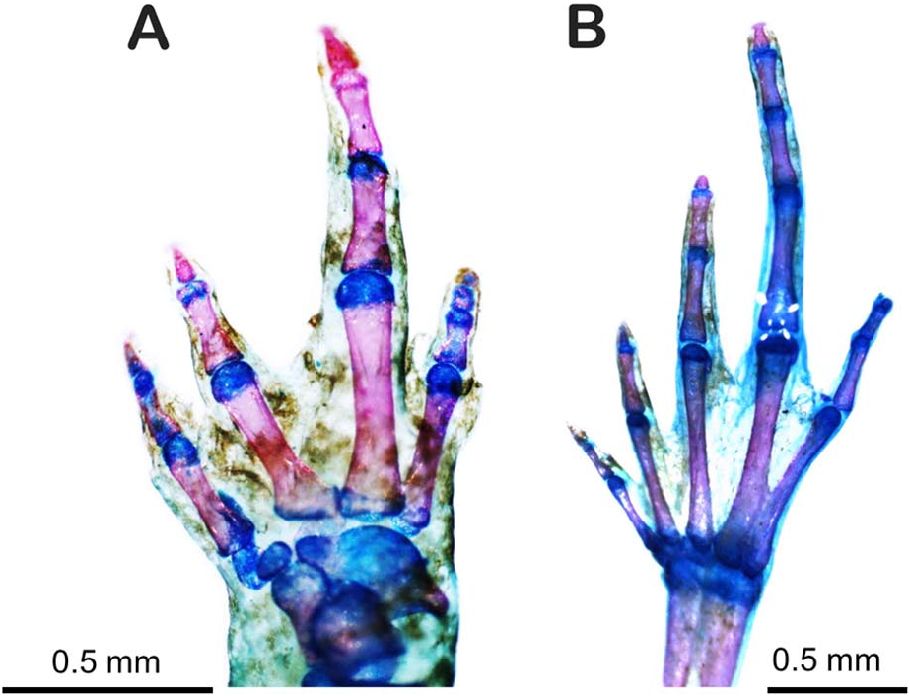
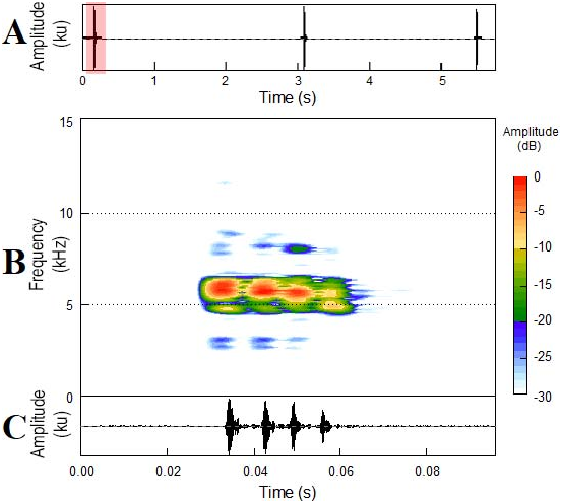
The new taxon is differentiated by the following combination of character states: (1) SVL less than 13.0 mm (males, 10.3–11.8 mm; females, 12.2–13.0 mm); (2) tympanum small, distinct with a visible membrane ( Fig. 1B View FIG ); (3) tympanic annulus present ( Fig. 1B View FIG ); (4) absence of a distinct glandular ridge that runs from the posterior part of the eye to the insertion of the forelimb ( Fig. 1B View FIG ); (5) dentigerous processes of vomers absent; (6) fingers without terminal discs or circumferential grooves, and fingers with mucronate tips ( Fig. 1C View FIG ); (7) toes with terminal discs or circumferential grooves and mucronate tips ( Fig. 1D View FIG ); (8) Finger IV with three phalanges ( Fig. 2A View FIG ); (9) subdigital pads present under fingers; (10) subarticular tubercles present under toes ( Fig. 1D View FIG and 3A View FIG ); (11) skin of dorsum smooth ( Fig. 3A View FIG ); (12) skin of belly smooth ( Fig. 3B View FIG ); (13) anal flap absent; (14) advertisement call composed of one note; (15) call note pulsed with side bands; and (16) high dominant frequency.
Phylogenetic analyses. —The consensus of the BI trees and best ML tree resulted in a similar topology supporting the monophyly of genus Adelophrŋne (ML bootstrap ¼ 95% and BI posterior probability ¼ 100%; Fig. 4 View FIG ). All populations of A. nordestina sp. nov. clustered together in a wellsupported clade (ML bootstrap and BI posterior probability ¼ 100%) inserted in the North Atlantic Forest Clade (NAFC; sensu Fouquet et al. 2012) with high support values (ML bootstrap and BI posterior probability ¼ 100%). The relationships among species within the NAFC were not always strongly supported. Adelophrŋne nordestina sp. nov. was recovered as sister to A. sp.2. (sensu Fouquet et al. 2012), but with nonsignificant support values in both inferences, and to form a clade with A. michelin (MI bootstrap ¼ 77% and BI posterior probability ¼ 99%). Adelophrŋne baturitensis and A. maranguapensis were recovered as sister species (MI bootstrap ¼ 79% and BI posterior probability ¼ 100) and sister to a clade containing A. nordestina sp. nov. þ A. sp 2 þ A. michelin ( Fig. 4 View FIG ). The uncorrected pairwise distances for the 16S rRNA gene within A. nordestina sp. nov. ranged between 3.5 and 3.6% ( Table 3 View TABLE ). The results of uncorrected pairwise distances of A. nordestina sp. nov. compared to other species of the genus ranged between 9.6 and 25.9%, being lowest between Adelophrŋne nordestina sp. nov. from the municipality of Caruaru, Pernambuco State, and A. michelin from the municipality of Igrapiuna, Bahia State ( Table 3 View TABLE ).
Description of the holotype. —Adult male, SVL 11.6 mm ( Fig. 1 View FIG ). Snout rounded, slightly triangular in dorsal view ( Fig. 1A View FIG ) and pointed, slightly rounded in lateral view ( Fig. 1B View FIG ). ETSD slightly larger than ED. END smaller than IND. Nostrils ovoid, not protruding. IND slightly smaller than IOD. Canthus rostralis indistinct, loreal region slightly concave. Choanae small, round, located laterally. Dentigerous processes absent. Tongue ovoid, free except for anterior margin. Pupil horizontally oval. Upper eyelid slightly convex. Temporal region vertical. Tympanum small, 35% of ED, annulus distinct and complete. Skin texture of venter, dorsum, and limbs smooth; flanks and ventral region of thighs areolate. Anal flap absent, cloacal opening horizontally positioned at slightly below the level of dorsal surface of thigh. Fingers without disks and with mucronate tips. Fingers thin, short, and without webbing. Relative finger lengths I<IV<II<III ( Fig. 1C View FIG ). Phalangeal formula 2–2–3– 3 ( Fig. 2B View FIG ). Fingers and palm surrounded by narrow strip of transparent skin. Subarticular tubercles absent, with round subdigital pads, formula 1–2–3–2, pads absent under ultimate phalanges and no supernumerary tubercles. Inner metacarpal tubercle ovoid. Outer metacarpal tubercle round, slightly larger than inner. Toes without webbing, cylindrical, slightly flattened. Relative toe lengths I<II<V<III<IV. Toes I, II, III, and IV with discs and mucronate tips; Toe V with circumferential grooves and without mucronate tips ( Fig. 1D View FIG ). Phalangeal formula 2–2–3–4–3 ( Fig. 2A View FIG ). Skin transparent on distal portion of toes. Subarticular tubercles present, formula 1–1–2–3–1, absent under ultimate phalanges. No supernumerary tubercles. Inner metatarsal tubercle oval. Outer metatarsal tubercle rounded, smaller than inner. Measurements are summarized in Table 2 View TABLE .
Color of the holotype. —In life, venter dark with many small orange and red dots; gular region with yellow and blue dots. Throat and underside of thighs and shanks orange. Dorsum orange with some dots. Upper eyelid and canthus rostralis gold. Loreal region dark brown, with a dark brown stripe extending along flank and reaching groin, with many small orange, red, and yellow dots. Thigh and tibia with numerous dark brown dots of various sizes. Iris reddish with black reticulations ( Fig. 3 View FIG ). In preservative, color pattern remains visible, but colors darken.
Variation. —The type series varies in dorsal coloration in life ( Fig. 5 View FIG ), ranging from black, brown, gold, to red. Some individuals have a dark brown interorbital V-shaped marking. Forelimbs reddish in some individuals. The coloration of the venter is dark and shows in some individuals scattered white or blue spots. Males are smaller than females (SVL of males, 10.3–11.8 mm; females, 12.2–13.0 mm). Morphometric variation is summarized in Table 2 View TABLE .
Etymology. —The name of the new species nordestina is a Portuguese feminine adjective meaning ‘‘from the northeast,’’ referring to its distribution in northeastern Brazil.
.
Comparisons with other species. — Adelophrŋne nordestina sp. nov. is distinguished from all other Adelophrŋne species, except A. baturitensis , by the presence of subarticular tubercles under the toes (subdigital pads in other species). Adelophrŋne nordestina sp. nov. is distinguished from A. glandulata , A. meridionalis , and A. michelin by having a distinct tympanum and tympanic annulus present (indistinct in the other species). Adelophrŋne nordestina sp. nov. is distinguished by having smaller body size (maximum SVL, 13.0 mm) than A. adiastola (maximum SVL, 14.0 mm), A. mucronata (maximum SVL, 14.9 mm), A. baturitensis (maximum SVL, 16.3 mm), A. gutturosa (maximum SVL, 16.0 mm), A. maranguapensis (maximum SVL, 17.4 mm), and A. patamona (maximum SVL, 23.0 mm). Adelophrŋne nordestina sp. nov. is distinguished from A. baturitensis , A. maranguapensis and A. patamona by the absence of discs or circumferential grooves on fingers (present in these species). The new species is further distinguished from A. glandulata and A. gutturosa by the absence of a distinct glandular ridge that runs from the posterior part of the eye to the insertion of the forelimb (present in these species). Adelophrŋne nordestina sp. nov. is distinguished from A. adiastola , A. baturitensis , A. maranguapensis , A. meridionalis , A. michelin , A. mucronata , A. pachŋdactŋla , and A. patamona by lacking dentigerous processes of vomers (present in these species). Adelophrŋne nordestina sp. nov. is distinguished from A. adiastola , A. glandulata , A. meridionalis , A. michelin , and A. pachŋdactŋla by having three phalanges in Finger IV (two phalanges in these species). Adelophrŋne nordestina sp. nov. is distinguished from A. adiastola , A. glandulata , A. mucronata , and A. patamona by having the dorsum with smooth skin (vs. shagreened to granular in A. adiastola , shagreened with small and rounded granules in A. glandulata , smooth with scattered small granules in A. mucronata , and tuberculated in A. patamona ). Adelophrŋne nordestina sp. nov. is distinguished from A. meridionalis by having Toes II, III, and IV with circumferential grooves and discs (vs. only Toe IV with circumferential grooves in A. meridionalis ). Adelophrŋne nordestina sp. nov. is distinguished from A. mucronata and A. maranguapensis by the absence of an anal flap (present in these species).
The advertisement call of 5 of the 10 recognized species in the genus Adelophrŋne has been described, namely, A. adiastola (erroneously reported as Phŋzelaphrŋne miriamae in Heyer 1977), A. gutturosa ( MacCulloch et al. 2008) , A. maranguapensis ( Cassiano-Lima et al. 2014) , A. mucronata ( Lourenço-de-Moraes et al. 2012), and A. patamona ( MacCulloch et al. 2008) . The advertisement call of A. nordestina sp. nov. differs from these other Adelophrŋne species by having the highest mean dominant frequency, with 5551 Hz (3200–5290 Hz in other species; Table 5). The advertisement call of A. nordestina sp. nov. differs from A. gutturosa , A. maranguapensis , and A. patamona by being formed of one single pulsed note (more than one note per call in these species). The call duration in A. nordestina sp. nov. (mean ¼ 0.042 ± 0.009 s) is shorter than that of A. adiastola (mean ¼ 0.24 s), A. gutturosa (mean ¼ 1.27 s), A. maranguapensis (mean ¼ 0.798 ± 0.159 s), and A. patamona (mean ¼ 0.63 s) but is longer than that of A. mucronata (mean ¼ 0.029.5 ± 0.004 s). The advertisement call of A. nordestina sp. nov. is most similar to that of A. mucronata because both are composed of one single note per call and have the dominant frequency above 5200 Hz, but the call of A. nordestina sp. nov. differs by having a pulsed call structure (nonpulsed in A. mucronata ).
| ESEC |
Entomological Society of Egypt |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Phyzelaphryninae |
|
Genus |
Adelophrnne nordestina
| Lourenço-De-Moraes, Ricardo, Lisboa, Barnagleison Silva, Oliveira Drummond, Leandro De, De Melo Moura, Carina Carneiro & Barbosa De Moura, Geraldo Jorge 2021 |
Adelophrŋne sp.
| Mesquita, D. O. & B. C. F. Alves & C. K. B. Pedro & F. G. R. Franca 2018: 459 |
Adelophrŋne baturitensis
| Fouquet, A. & D. Loebmann & S. Castroviejo-Fisher & M. T. Rodrigues 2012: 550 |
| Loebmann, D. & V. G. O. Dill & C. F. B. Haddad 2011: 75 |