Sertania Callaghan & Kaminski, 2017
|
publication ID |
https://doi.org/10.11646/zootaxa.4312.1.8 |
|
publication LSID |
lsid:zoobank.org:pub:B4C23258-B83E-44Ba-80C3-Fbf07Aa81311 |
|
DOI |
https://doi.org/10.5281/zenodo.6040422 |
|
persistent identifier |
https://treatment.plazi.org/id/68B2D520-8732-41BD-9C9E-BDE8110EF7EB |
|
taxon LSID |
lsid:zoobank.org:act:68B2D520-8732-41BD-9C9E-BDE8110EF7EB |
|
treatment provided by |
Plazi |
|
scientific name |
Sertania Callaghan & Kaminski |
| status |
gen. nov. |
Sertania Callaghan & Kaminski , gen. nov.
Type species: Hamearis guttata Stichel, 1910 , here designated.
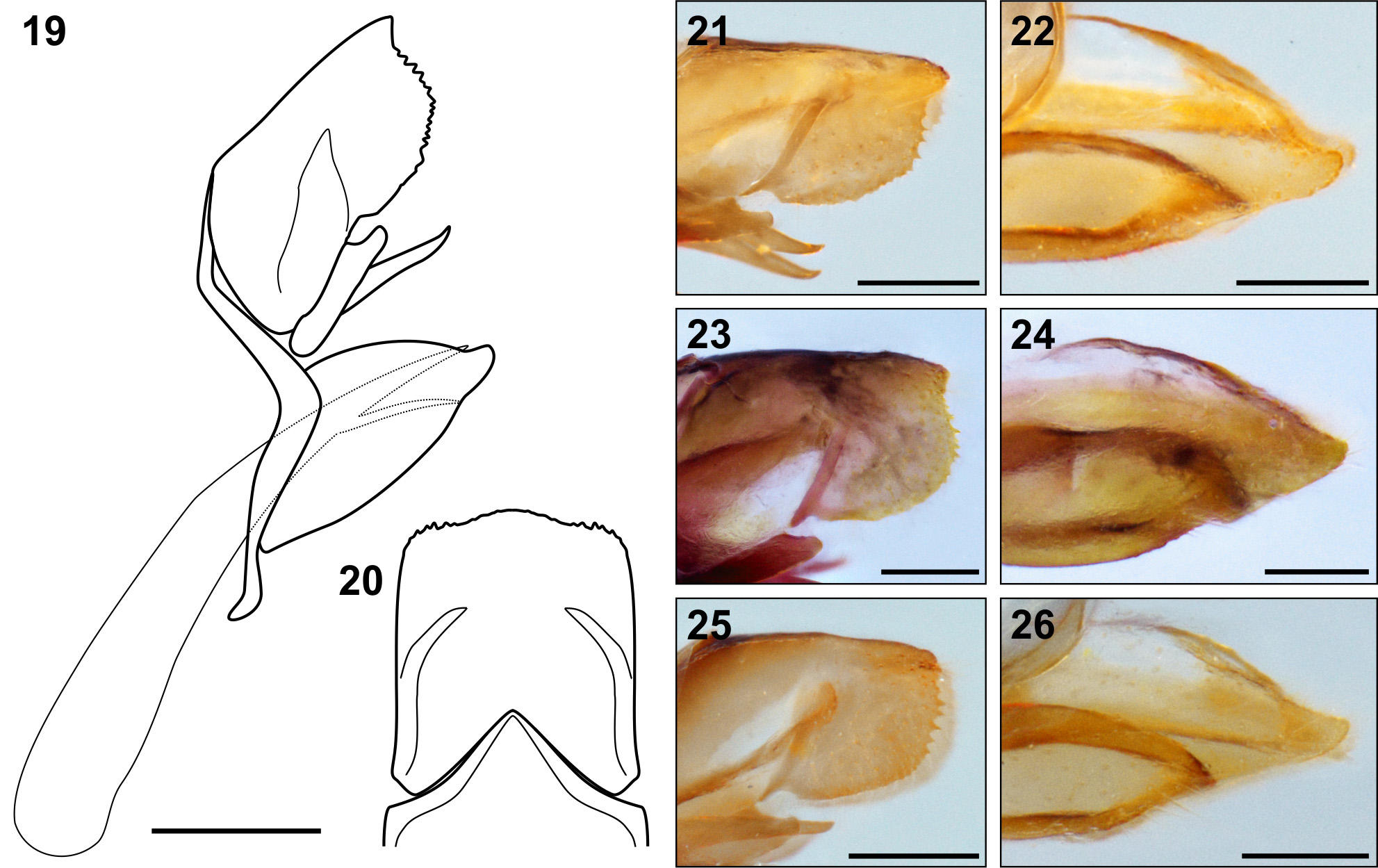
Diagnosis. The small size, wing shape and color pattern of adults ( Figs 1–12 View FIGURES 1 – 12 ) make them similar to small species of sympatric Aricoris (Nymphidiini) , but the male genitalia and the lacking of the spot at the base of the HW cell Sc + R1 distinguish species of Sertania gen. nov. from all known Aricoris species. The following two characters suggest that Sertania gen. nov. is related to the tribe Riodinini ( sensu Harvey 1987): 1) In FW, veins R1 and R2 emerge before the end of the FW discal cell (present in all tribe except Isapis Doubleday, 1847 (Stichel 1911)); and 2) male genitalia with a broad strap-like pedicel reaching to the end of aedeagus where it joins a broad plate serving to support the aedeagus, and which returns along base of valvae to near the projection of saccus short. However, the wavy distal margin of FW present in Sertania gen. nov. is rare in Riodinini (reported in some species in distant genera as Baeotis , Lasaia Bates, 1868 and Amphiselenis Staudinger, 1888 ). Finally, the deeply notched anterior margin of the tegument with the sides of the indentation parallel, stated as the main synapomorphy of Riodinini ( Harvey 1987) , is a shallow indentation in Sertania gen. nov. ( Fig. 20 View FIGURES 19 – 26 ). This combination of characters is enough to distinguish the new genus from all other described genera of Riodinidae .
Etymology. The generic name refer to the Portuguese word “sertão” [sɛʁˈtɐ̃w], ̃ meaning a “place in the interior, far from the sea”, possibly an abbreviation of “desertão” derived from the Latin form desertus (wilderness). Currently, it is used to indicate the dry remote areas (cerrados and caatingas) in the interior of Brazil (Antonio-Filho 2011).
Description. Male ( Figs 13–14 View FIGURES 13 – 18 , 19–22 View FIGURES 19 – 26 ): Wing shape: FW costa slightly indented midway, then slightly arched to a pointed apex, distal margin slightly indented to M2, then strongly indented to Cu1, then curved convex to origin to Cu2 then slightly indented to tornus; inner margin slightly indented. HW costa straight to Sc, distal margin curved to Cu2 where it is indented before anal angle; inner margin slightly concave to base. Venation: FW with four radial veins, R2 arises basad of the discal cell; 3A of HW slightly truncated, reaching inner margin closer to base; humeral vein truncated.
Dorsal surface: Ground color of both wings dark brown. FW discal cell with two irregular, white spots outlined by dark scales, with two similar white spots below in cell Cu2-2A; distad in post-medial area an irregular median row of rounded white spots edged basally with black, one each occupying cells R1-R2 to Cu2-2A in which there are two; each bordered basally in black; distally the post-medial area with a submarginal row of smaller orange/white spots between the veins; margin with orange or black scaling; fringe black except at the end of cells Cu1-Cu2 and Cu2-2A where it is white. HW with two white spots outlined with black in the discal cell with two additional spots below in cell Cu2-2A and one smaller white spot above in cell Sc-R s; post-medial area with a median row of faint, irregular white spots from Sc-Rs to Cu1-Cu2, one in each cell, and two in Cu2-2A; distally a submarginal band of orange spots in cells R s to 2A, bordered distad in black; margin with variable black and orange scaling; fringe variably white and black.
Ventral surface: Ground color of both wings brown, variably infused with orange and white scaling. FW dorsal surface maculation reflected ventrally, the white spots in discal area bordered basad and distad with black lines, those in post-medial area borded basad in black; veins in post-medial area outlined in red scaling; submarginal row of spots largely obliterated, though variably marked with red; distal margin bordered in red/black; fringe as dorsally. HW discal area and anal margin to end of vein 2A strongly infused with white scaling interspersed with red scales, veins outlined in red; post-medial area dark brown with increasing infusion of white scaling toward the margin; veins variably outlined in red; margin red; fringe as dorsally.
Head: Eyes brown, frons white with two reddish brown patches of scales, upper surface and collar orangebrown; labial palpi with long white/light brown scales on first two segments, third segment light brown, 60% length of second; antennae brown with white at segments and at base of elongated clubs, antennal length 75% of forewing length.
Body: Dorsal surface of thorax black, ventral surface light brown, appendages white; forelegs truncated with pubescent white scaling, tibia unimerous, coxa elongated and pointed, midlegs and hindlegs with long spurs at joints and scattered spines along inner margin of tarsal segments. Abdomen dorsally and laterally brown with variable red scaling, ventrad white with long white scales around genitalia.
Genitalia: Uncus bipartite with lateral lobs widely separated and margin serrated, in lateral view rounded; tegumen extending between lobs of uncus with slight peak caudad, tegumen equals length of uncus, posterior margin deeply indented, but not notched; socii broad, pointed, with cilia, and long, reaching cephalad past margin of tegumen; gnathos long, pointed, doubled inwardly; vinculum joined around margin of indentation of tegumen, wider in middle with a small internal flange, slightly curved to back of valvae, saccus only a slight widening at the base; vinculum connected to membrane of 8th sternite supporting long scales; valvae simple, rounded cephalad, pointed caudad, with tips flaired outward, unfused basad and fused posteriad on dorsal surface; aedeagus long, broad with blunt tip without cornutus; attached to this a broad strap-like pedicel reaching to end of aedeagus where it joins a broad plate serving to support aedeagus, which returns along base of valvae to near the vinculum.
Description. Female ( Figs 15 View FIGURES 13 – 18 , 27–32 View FIGURES 27 – 32 ): The female differs from the male in the following: Wing shape: FW costa straight with apex more rounded. Dorsal wing surface: Ground color of both wings lighter brown and infused with variable lighter orange-yellow scaling. FW with the size of the white spots much larger and infused with variable yellow scaling; submarginal area infused with light orange scaling penetrating to the margin along the veins, breaking the dark brown scaling into a series of broad spots between the veins; margin dark red. HW with greater infusion of yellow-orange scaling; discal and post-medial spots much larger with median row nearly joined into a solid light orange band bordered distally and ventrally with dark brown; distal margin and submarginal areas light orange enclosing a row of dark brown spots between the veins; inner margin grey-brown; distal margin and fringe black. Ventral surface: Ground color of both wings lighter, maculation of dorsal surface reflected ventrally identical but more pronounced. FW discal area light orange, post-medial area with strong infusion of dark red scaling; submarginal area grey, margin red; fringe as on dorsal surface. HW with strong infusion of white scaling in the discal area, nearly obliterating white spots; post-medial area with mottled red/white scaling, veins outlined in red; margin red; fringe white/black.
Head: White frons containing reduced brown scales.
Body: Dorsal surface color of thorax and abdomen light brown, ventral surface and appendages white; forelegs with longer spines at joints in tibia, smaller spines on inner margin of tarsal segments.
Genitalia: Corpus bursae with two large, parallel invaginate signa, with wide, sharp points located cephalad in corpus bursae, ductus bursae very long, entering ostium bursae slightly from left, ostium bursae with rounded base to which the narrow ductus seminalis is attached, narrowing to the sinus vaginalis with dense sclerotization laterally; lamella postvaginalis with sclerotized hole in middle; papillae anales somewhat pointed, rounded when viewed laterally. Spermatophores encountered in the corpus bursae large and with long tails occupying ductus bursae; never more than one per female.
History of classification. Hamearis guttata was described by Stichel (1910) from a single female originating from Mendoza, Argentina, with the specimen number 3561. The type is deposited in the collection of the Humboldt Universität, Berlin (examined). The next year, Giacomelli (1911) described Apodemia minuscula from La Rioja, Argentina. This was followed by the description of Hamearis guayapensis Köhler (1923) from Guayapa, La Rioja, Argentina. Both of these taxa were synonymized with H. guttata in the same year by Stichel (1928) and Köhler (1928), respectively, and followed by Stichel (1930) in the Lepidopterorum Catalogus. All Neotropical species described in Hamearis , including guttata , were transferred to Audre by Hemming (1967). Callaghan & Soares (2001) described the subspecies Audre guttata jaibensis from a newly discovered population in northern Minas Gerais, Brazil. The following year, Hall & Harvey (2002a) removed guttata provisionally to the genus Emesis . Finally, Jauffret et al. (2008) described an additional subspecies, “ Emesis ” guttata lambedor , from the state of Ceará, Northeastern Brazil.
Systematic position. T he systematic position of H. guttata was never solved based on adult morphology only. This taxon was initially associated with the genera Hamearis and Audre due to its alar maculation, wing shape and distribution range, being externally similar to several species currently in Aricoris . In a phylogenetic study of the Lemoniadina, Penz & DeVries (1999) examined specimens of H. guttata , concluding that it did not belong in Audre , but did not propose an alternative. Callaghan & Soares (2001) reached the same conclusion based on morphology, but likewise did not propose an alternative classification. Later, in a phylogenetic study of Nymphidiini , Hall & Harvey (2002a) removed H. guttata from Audre and assigned it to Emesis based on undetailed similarities of the male genitalia, suggesting that it should be related to Emesis xanthosa ( Stichel, 1910) (previously removed from Charis Hübner , [1819] by Hall & Harvey 2002b). However, there is not enough information to support the inclusion of E. xanthosa in Sertania gen. nov. This species was described from a single female with no locality data; males are unknown to date. The female genitalia of E. xanthosa is considerably different from that of Sertania gen. nov. The male specimen of E. xanthosa referred in Hall & Harvey (2002a, b) was not located in the collections at the National Natural History Museum (Smithsonian) in Washington D.C. Until a male is located or a fresh individual be available for molecular work, the position of E. xanthosa and its relationship with Sertania gen.
nov. will remain a mystery. In summary, morphological characteristics do not permit the inclusion of Sertania gen. nov. in any of known Riodinidae tribe. In addition, molecular data given by Seraphim et al. (in press) indicate that Sertania gen. nov. is a unique lineage with a very long branch with uncertain affinities to the tribes Riodinini , Symmachiini and Helicopini , justifying the description of a new genus for this clade (for a similar situation in Euptychiina see Freitas et al. 2016).
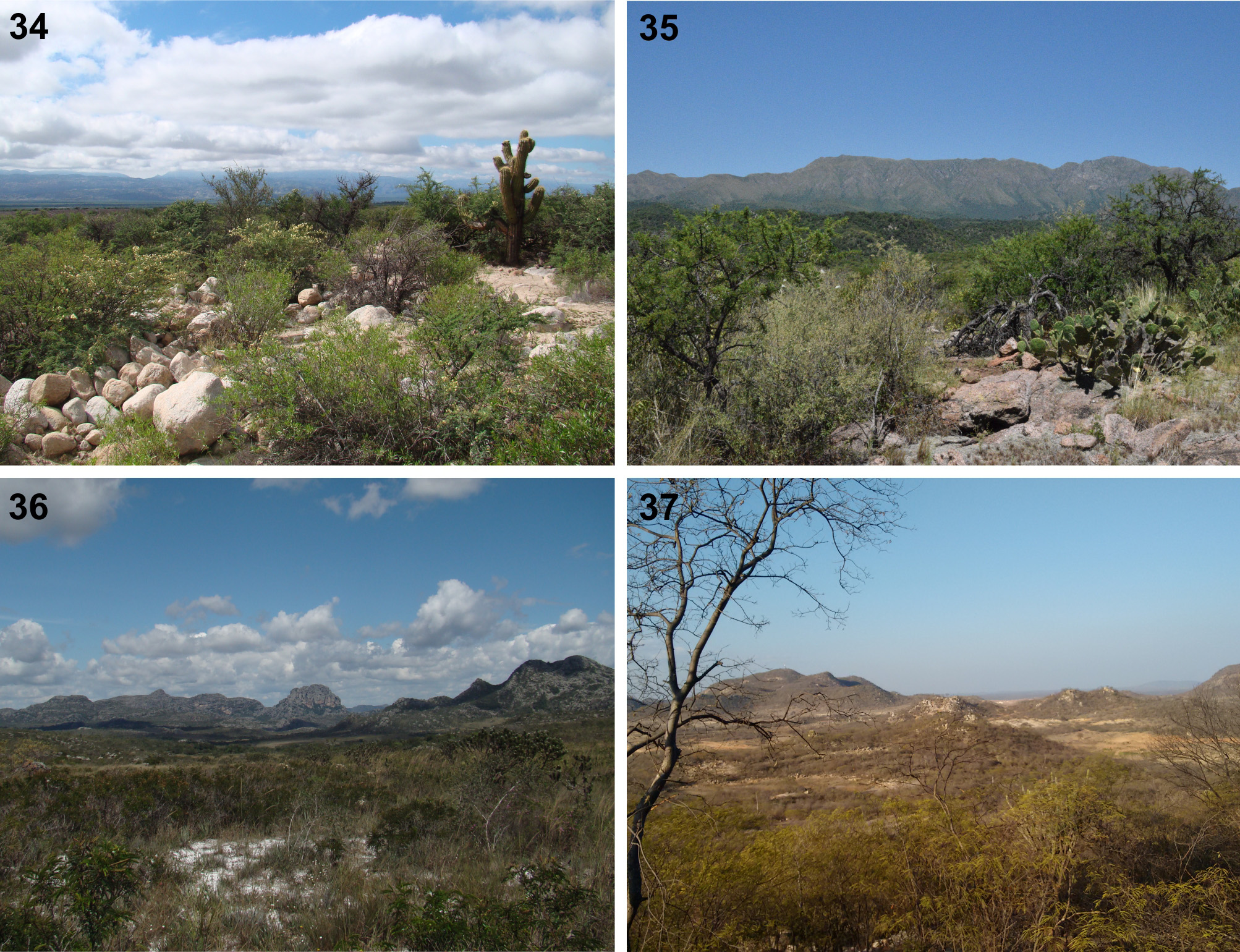
Biology and distribution. Sertania gen. nov. is known from open dry vegetal formations (namely the south American ‘dry diagonal’, Figs 33–37 View FIGURE 33 View FIGURES 34 – 37 ), and has been recorded in two dry vegetal physiognomies from Argentina and Paraguay (known as “monte” and “chaco”), in the dry forests of northeast Brazil (known locally as “caatinga”), and rocky high altitude grasslands (known locally as “campos rupestres”) in Minas Gerais (SE Brazil). Host plants and immature stages are unknown. A note on an old specimen of S. guttata comb. nov. in the museum at La Plata, Argentina reads “con crematogastor” (meaning “with Crematogaster [ants]”), but the real importance of this note is unknown, and ant-association (myrmecophily) cannot be assumed. That would be intriguing, as no myrmecophilous relationships are known among the closely related lineages in incertae sedis and Riodinini ( DeVries 1997, Kaminski 2008).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |