Alopex lagopus (Linnaeus, 1758)
|
publication ID |
https://doi.org/10.5281/zenodo.6331155 |
|
DOI |
https://doi.org/10.5281/zenodo.6335049 |
|
persistent identifier |
https://treatment.plazi.org/id/03ACCF40-BF2E-FFD3-7BA2-F972F749DFFB |
|
treatment provided by |
Conny |
|
scientific name |
Alopex lagopus |
| status |
|
Arctic Fox
French: Renard arctique / German: Polarfuchs / Spanish: Zorro artico
Other common names: Polar Fox
Taxonomy. Canis lagopus Linnaeus, 1758 View in CoL ,
Lapland, Sweden.
Sometimes placed as subgenus of Vulpes or Canis . The most closely related species are V. velox and V. macrotis , neither of which occur in the tundra. Four subspecies recognized.
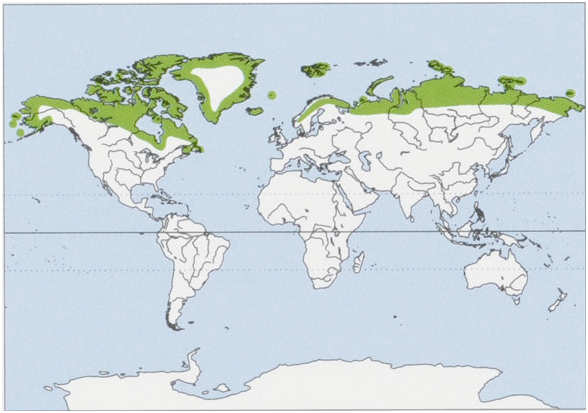
Subspecies and Distribution.
A. l. lagopus Linnaeus, 1758 — most of the circumpolar range, in all Arctic tundra habitats.
A. l. beringensis Merriam, 1902 — Russia (Commander Is).
A. l. fuliginosus Bechstein, 1799 — Iceland, Greenland, Svalbard.
A.l. pribilofensis Merriam, 1902 — Alaska (Pribilof Is). View Figure
Descriptive notes. Head-body 55-75 cm (males), 50-65 cm (females), tail 28-42- 5 cm for males and 25- 5-32 cm females; weight 3-58- 4-23 kg for males and 3-14- 3-69 kg for females. Males are slightly larger than females. A small fox with rather short legs and a long fluffy tail. Thick and soft winter fur with dense underfur and long guard hairs. Occurs in two distinct color morphs, “blue” and “white”. Both morphs change seasonally: “blue” molts from chocolate brown in summer to paler brown tinged with blue sheen in winter. In winter, the “white” morph is almost pure white with a few dark hairs at the tip of the tail and along the spine; in summer,it is brown dorsally and pale gray to white on its underside. Color morphs are determined genetically at a single locus, “white” being recessive. The “blue” morph comprisesless than 1% of the population throughout most of its continental range, but comprises 25-30% in Fennoscandia ( Norway, Sweden, and Finland) and 65-70% in Iceland. The proportion of “blue” morphs also increases in coastal areas and on islands, where it can reach up to 100% (e.g. Mednyi Island, Russia; St. Paul Island, Alaska). Within each morph, there is considerable variation in appearance, which seems to be independent of the genetic locus for color morph. In Sweden, there occasionally are sand-colored foxes in summer, but they appear to be of the “white” morph without brown pigment, and in Iceland, cinnamon colored foxes of both the white and blue color morph occur. The dental formulais13/3,C1/1,PM 4/4, M 2/3 = 42.
Habitat. Arctic and alpine tundra of Eurasia, North America, and the Canadian archipelago, Siberian islands, Greenland, inland Iceland, and Svalbard. Sub-Arctic maritime habitat in the Aleutian island chain, Bering Sea Islands, Commander Islands, and coastal Iceland.
Food and Feeding. The Arctic Fox is an opportunistic predator and scavenger. In most inland areasit is heavily dependent on fluctuating rodent populations. Main prey include Lemmings, both Lemmus spp. and Dicrostonyx spp. In Fennoscandia, L. lemmus was the main prey in summer (85% frequency of occurrence in feces) followed by birds ( Passeriformes, Galliformes, and Caridriiformes , 34%) and Reindeer (Rangifer tarandus, 21%). In winter, ptarmigan and grouse ( Lagopus spp.) are common prey, in addition to rodents and Reindeer. Changes in Fox populations have been observed to follow those of their main prey in three- to five-year cycles. Foxes living near icefree coasts have access to inland prey and also sea birds, seal carcasses, fish, and invertebrates connected to the marine environment, leading to relatively stable food availability and a more generalist strategy. In late winter and summer, foxes found in coastal Iceland feed on seabirds (Uria aalge, U. lomvia), seal carcasses, and marine invertebrates. Inland foxes rely more on Ptarmigan in winter, and migrant birds, such as geese and waders, in summer. In certain areas, foxes rely on colonies of arctic geese, which can dominate their diet locally. Arctic Foxes forage singly, presumably the most efficient foraging technique in view of the species’ main prey base of rodents and birds. When food is abundant, they cache food forlater use. Caches can contain single prey items or a variety of items, and sometimes include lemmings or goose eggs.
Activity patterns. Arctic Foxes remain active year round. They are primarily nocturnal, but exhibit flexible activity patterns, often in accordance with main prey species.
Movements, Home range and Social organization. The basic social unit of the Arctic Fox is the breeding pair. Both parents take an active part in rearing the pups. For the first three weeks after birth, the pups are mostly dependent on milk and the female rarely leaves the natal den. During this time, the male provides food for the female. As meat increasingly forms a larger proportion of the pups’ diet, the roles of the parents become more similar and the female takes an active part in hunting and provisioning the pups. Non-breeding foxes, usually yearlings from the previous litter, may help. Supernumerary females generally emigrate before the pups attain independence of the den at 8-10 weeks. However, on Mednyi Island, there are permanent Arctic Fox groups comprising up to six adults, and complex social systems have also been observed on otherislands. Temporary groups of non-breeding individuals are also sometimes formed. Arctic Foxes normally are strongly territorial when breeding, with natal dens generally used by only one family group. Breeding pairs remain together in the same territory and use the same natal den for up to five years. Territories may include more than a single breeding pair, and closely related breeding pairs may even share a den. Home ranges in inland areas vary with lemming abundance ( 15-36 km?), but generally are smaller in coastal habitats (e.g. 5-21 km? in Alaska). On Svalbard, home range sizes range from 10 km? to as large as 125 km ®. Home ranges of group members generally overlap widely with each other, but very little with those of neighboring groups. Scent marking of territories with urine is common. Vocalizations and postures such as an erected tail to attract the attention of conspecifics are common during territory disputes. In Alaska, seasonal migrations are reported when individuals leave breeding grounds in autumn, travel to the coast, and return in late winter or early spring. Large-scale emigrations have been recorded in Canada, Fennoscandia, and Russia. These may result from drastic reductions in food supplies, such as a population crash in lemmings.
Breeding. Mating occurs between February and May and births take place from April to July. Gestation lasts 51-54 days and pup weight at birth is 80-85 g, but may be lower in larger litters. Litter size varies with food availability, being larger in areas with rodents. Recorded mean litter sizes at weaning varied from 2-4 (St. Paul Island) to 7-1 ( Russia). On WrangelIsland, Russia, in years with high lemming abundance, up to 19 pups per litter have been observed. The ability of Arctic Foxes to produce large litters is facilitated by their access to large and relatively safe dens. Den sites are large, with complex burrow systems, and the largest dens are preferred for breeding. These may have up to 150 entrances and are usually situated on elevated mounds, river banks or ridges. Good denning sites lie above the permafrost layer, accumulate comparatively little winter snow and are sun-exposed, often facing south. The average lifespan of dens in the Canadian tundra has been estimated at 330 years. Some are used repeatedly, year after year, others infrequently. Pup rearing is confined to the snow-free period from June to September, after which the young gradually become independent. Lactation generally lasts 8-10 weeks. Growth rate from weaning in early July to late August has been recorded at 30-34 g /day. Foxes reach sexual maturity at ten months.
Status and Conservation. CITES not listed. Classified as Least Concern on The [UCN Red List. The Arctic Fox, however, is regionally threatened in Sweden (“endangered”), Finland (“critical”) and mainland Norway (“endangered”). In 1983, following the introduction of mange caused by ear canker mites (Otodectes cynotis) transmitted by dogs, the Mednyi Island foxes were listed in the Russian Red Data Book. The world population of Arctic Foxes is on the order of several hundred thousand animals. In most areas, population status is believed to be good. The species is common in the tundra of Russia, Canada, coastal Alaska, Greenland, and Iceland. Exceptions are Fennoscandia, Mednyi Island ( Russia), and the Pribilof Islands, where populations are at critically low levels. On the Pribilof Islands, fox populations appear to be declining further. In most of its range, the Arctic Fox is not protected. However, the species and its dens have received total legal protection in Sweden since 1928, in Norway since 1930, and in Finland since 1940. In Europe, the Arctic Fox is a priority species under the Actions by the Community relating to the Environment (ACE) and is therefore given full protection. On St. Paul Island the declining population currently has no legal protection. In Norway ( Svalbard), Greenland, Canada, Russia, and Alaska, trapping is limited to licensed trappers operating in a defined trapping season. The enforcement of trapping laws appears to be uniformly good. In Iceland, bounty hunting takes place over most of the country outside nature reserves. Hunting for fur has long been a major mortality factor for the Arctic Fox; however, the decline of the fur industry has reduced the threat of over-exploitation for most populations. In some areas gene swamping by farm-bred Blue Foxes may threaten native populations. There can also be indirect threats such as diseases and organochlorine contaminants, or direct persecution, as occurs on St. Paul Island. Misinformation as to the origin of Arctic Foxes on the Pribilofs continues to foster negative attitudes and the long-term persistence of this endemic subspecies is in jeopardy.
Bibliography. Angerbjorn, Hersteinsson & Tannerfeldt (2004), Angerbjorn, Stroman & Becker (1997), Angerbjorn, Tannerfeldt, Bjarvall et al. (1995), Angerbjorn, Tannerfeldt & Erlinge (1999), Audet et al. (2002), Chesemore (1975), Dalerum et al. (2002), Eberhardt, Garrott & Hanson (1983), Eberhardt, Hanson et al. (1982), Eimhagen et al. (2000), Frafjord (1994), Frafjord & Kruchenkova (1995), Frafjord & Prestrud (1992), Garrott & Eberhardt (1982, 1987), Hersteinsson (1984), Hersteinsson & Macdonald (1982, 1992, 1996), Hersteinsson et al. (1989), Kaikusalo & Angerbjorn (1995), Macpherson (1969), Nasimovic & Isakov (1985), Ovsyanikov (1993), Prestrud (1992a, 1992b, 1992¢), Samelius & Lee (1998), Tannerfeldt & Angerbjorn (1998).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.