Cameraria Chapman, 1902
|
publication ID |
https://doi.org/10.11646/zootaxa.3594.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:B00799F3-F397-438C-B1E1-A8440E636921 |
|
DOI |
https://doi.org/10.5281/zenodo.6308530 |
|
persistent identifier |
https://treatment.plazi.org/id/03ADE350-B131-FFAF-F1CF-FBE58A19CA80 |
|
treatment provided by |
Felipe |
|
scientific name |
Cameraria Chapman, 1902 |
| status |
|
Cameraria Chapman, 1902 View in CoL
Cameraria Chapman 1902: 141 View in CoL .
Type species: Lithocolletis guttifinitella Clemens, 1859 by original designation.
Historic account. The genus Cameraria was proposed by the British author T. A. Chapman (1902) during a presentation at the Entomological Society of the City of London. However, as pointed out in his talk, Chapman did not study the species himself, but relied on Chambers’ observations and named the genus after him (chamber = camera in Latin). The designation of the new genus was based solely on larval morphology: Chambers divided mining Gracillariidae larvae into two groups: ‘cylindrical-larva group’ (group I), and ‘flat-larva group’ (group II). Annette F. Braun (1908) still called ‘flat’ mining larvae Lithocolletis , but she assigned them to Group II following Chambers’ observations. She added another character which diagnoses this Lithocolletis group II—the apically edged white markings of the forewing. The year after Braun’s publication, Busck (1909) praised Braun’s (1908) work and corrected the generic arrangement she had proposed. He pointed out that “these two [ Phyllonorycter and Cameraria ] branches developed parallel… there can clearly not have been any crossings between [them] ... neither could one have been developed from the other.” Therefore, Busck (1909) suggested the recognition of the two groups or branches of mining moths as separate genera - Phyllonorycter and Cameraria . In the same year, Braun (1909) presented a detailed account on the morphological structures of the larval heads of both cylindrical and flattened forms, and assigned the latter group as Cameraria . Furthermore, Braun (1909) presented her own diagram illustrating the probable relationship of Cameraria and Phyllonorycter . The flat nature of the larva and the persistence of a sap-feeding habit throughout the entire feeding period in Cameraria remained a unique characteristic that was recognized by some North American microlepidopterists at the beginning of the 20th century, and the usage of Cameraria was firmly established in their publications ( Braun 1909, 1914; Busck 1909; DeGryse 1916). Mosher (1916) recognized another unique feature of Cameraria — the absence of a distinct cremaster at the caudal end of the pupa. C. R. Ely (1918) revised the North American Gracillariidae on the basis of wing venation, pointing out that Phyllonorycter and Cameraria both possess 7 veins [remark: Ely excluded the rudimentary CuP in his counting] and rough head. Following the above-mentioned lepidopterists, he defined Cameraria on the basis of the flat larva and mines -“always on the upper side of the leaf of the food plant.” Despite the frequent publications on Cameraria in North American entomological;literature at the beginning of the last century, the genus was not recognized as distinct by other lepidopterists of that time. For instance, Fletcher (1929) treated Cameraria as a synonym of Lithocolletis , and the debate continued until the 1960s. In 1961, two important publications appeared concerning Lithocolletinae genera: one by Vári (1961) and the other by Kumata (1961). Vári (1961: 206) followed Fletcher and treated Cameraria as a synonym of Lithocolletis . However, much later, he ( Vári et al. 2002: 26) excluded Cameraria from the synonymy of Phyllonorycter . We highly value the note of Vári (1961: 207): “I do not attempt at the present stage to erect new genera for these species [presented by him as Lithocolletis ] as the male genitalia cannot be arranged in corresponding groups.” Kumata (1961) described the genus Chrysaster and in his differential diagnosis compared it with two separate genera: Cameraria and Lithocolletis . A robust treatment of East Palaearctic Cameraria followed a couple of years later ( Kumata 1963). Until recently Cameraria was considered as a North American genus found in Asia as well. Papers on Cameraria biodiversity, ecology, biology and evolutional relationships were published by North American microlepidopterists ( Hinckley 1971, 1972; Opler 1971, 1974; Opler & Davis 1981; Faeth 1990a,b, 1991a,b; Bultman & Faeth 1985, 1986a,b,c, 1987, 1988; Maier & Davis 1989 etc.). Cameraria was also recorded from the Russian Far East and Central Asia ( Ermolaev 1979; Noreika & Puplesis 1992; Noreika 1994, 1997). In Europe, little work was conducted on Cameraria until Deschka & Dimić (1986) described Cameraria ohridella feeding on Horse-Chestnut ( Aesculus hippocastanum ) from Macedonia. This species quickly became a highly invasive pest in West and Central Europe. In response to the invasion of this species into Europe, a plethora of publications on Cameraria ohridella (no less than 25 papers per year) appeared in the European entomological literature during the period of 1990–2008. The name Cameraria was even taken over as a common word by the mass media, and C. ohridella became the most studied lepidopteran species ever ( Lees et al. 2011). Despite the attention that was drawn to this species, the diagnostic characters of tropical Cameraria and the phylogenetic position of the genus within Lithocolletinae remained unclear. There were significant attempts made to review the ecology, host-use evolution, and biogeography of Cameraria species groups ( Opler & Davis 1981), the morphology of adults and larval instars ( Kumata 1961, 1963, 1993, 1995), and some general observations on morphological characters for taxonomic and diagnostic purposes ( Davis 1987; Skuhravý 1998; De Prins et al. 2003; Johne et al. 2005), as well as some cytogenetical peculiarities ( Puplesiene & Noreika 1993; De Prins et al. 2002). Furthermore, since C. ohridella is a devastating pest on A. hippocastanum , a very popular ornamental plant in parks and avenues in Europe, many studies focused on understanding the chemical communication (pheromones) of this species in order to control it ( Szöcs & Toth 1998; Svatoš et al. 1999, 2001, 2009; Szöcs et al. 2000, 2006; Francke et al. 2002; Kindl et al. 2002; Mircheva & Subchev 2002, 2003; Kalinová et al. 2003; Raspotnig et al. 2003; Augustin et al. 2004). However, these specialized studies were not focused on other species in the genus. The authors of the present study made several unsuccessful attempts to attract Afrotropical Cameraria using sex attractants synthesized for C. ohridella (product number 008524, Biobest N.V. Belgium) and Phyllonorycter ( De Prins et al. 2009) , and concluded that Afrotropical Cameraria species are not attracted to C. ohridella lures, which are highly effective in Europe. This presumably indicates that C. ohridella and its Afrotropical congeners are distantly related. Molecular studies on Cameraria mainly aimed to demonstrate the invasive aspect of C. ohridella ( Perny 1997; Kovács et al. 2000; Lakatos et al. 2003; Hernandez-Lopez et al. 2009; Péré et al. 2010) and to clarify the origin of this species ( Valade et al. 2009).
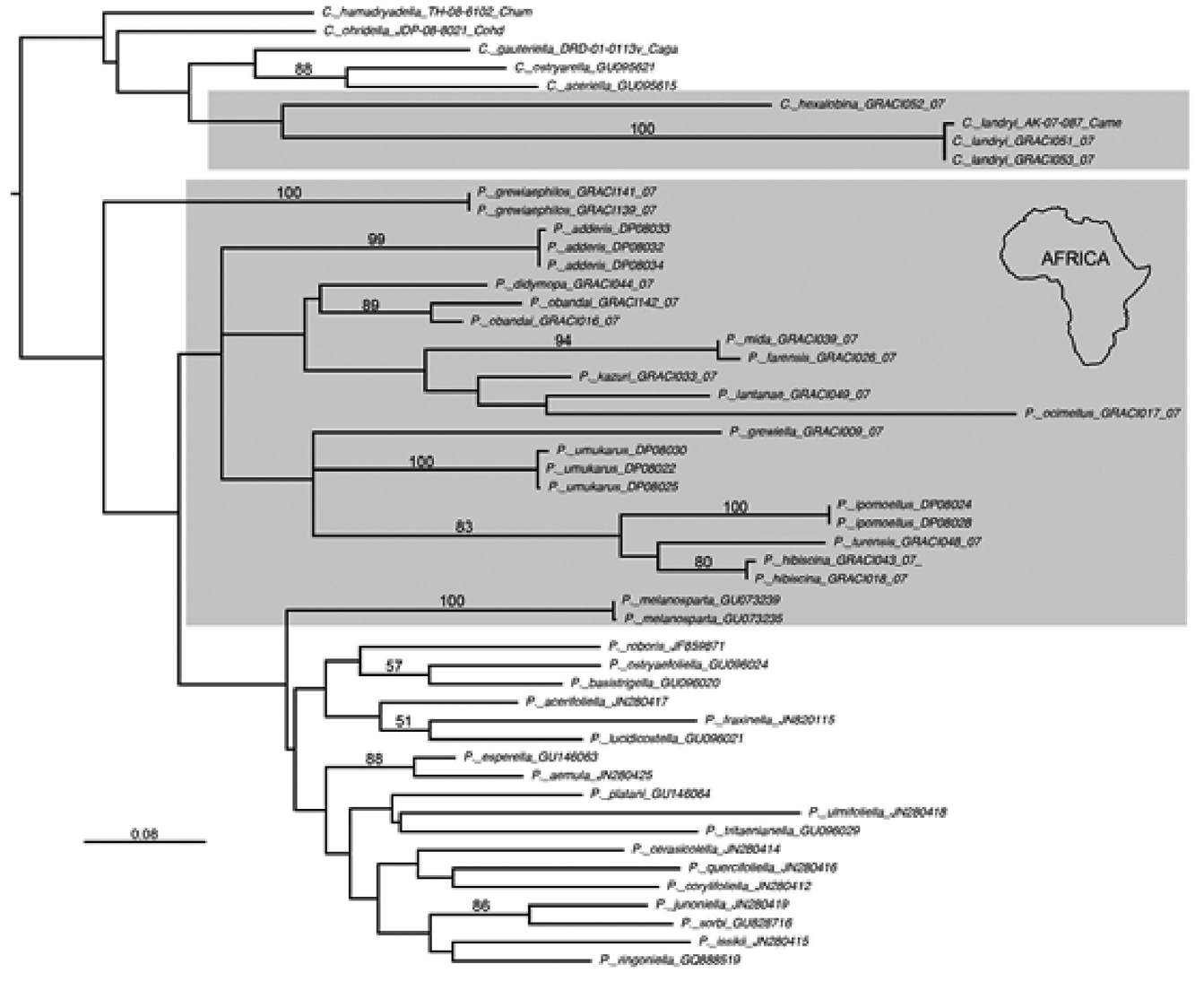
Cameraria is distributed worldwide, but has thus far been presumed to be localized to temperate regions: the greatest species richness of Cameraria is found in the Nearctic region (53 species), then followed by the Oriental (12 species) and Palaearctic (7 species) regions. Cameraria is not recorded from the Neotropical and Australian regions. It was not known from the Afrotropical region either until the assumption ( van Nieukerken & De Prins 2007) that an Annonaceae-feeding species in southern Africa “in fact also belongs to Cameraria ”, thus constituting the first record of this genus for the Afrotropical region. Here we present eight Afrotropical species accommodated into the genus Cameraria , confirming the presence of this genus in the Afrotropics.
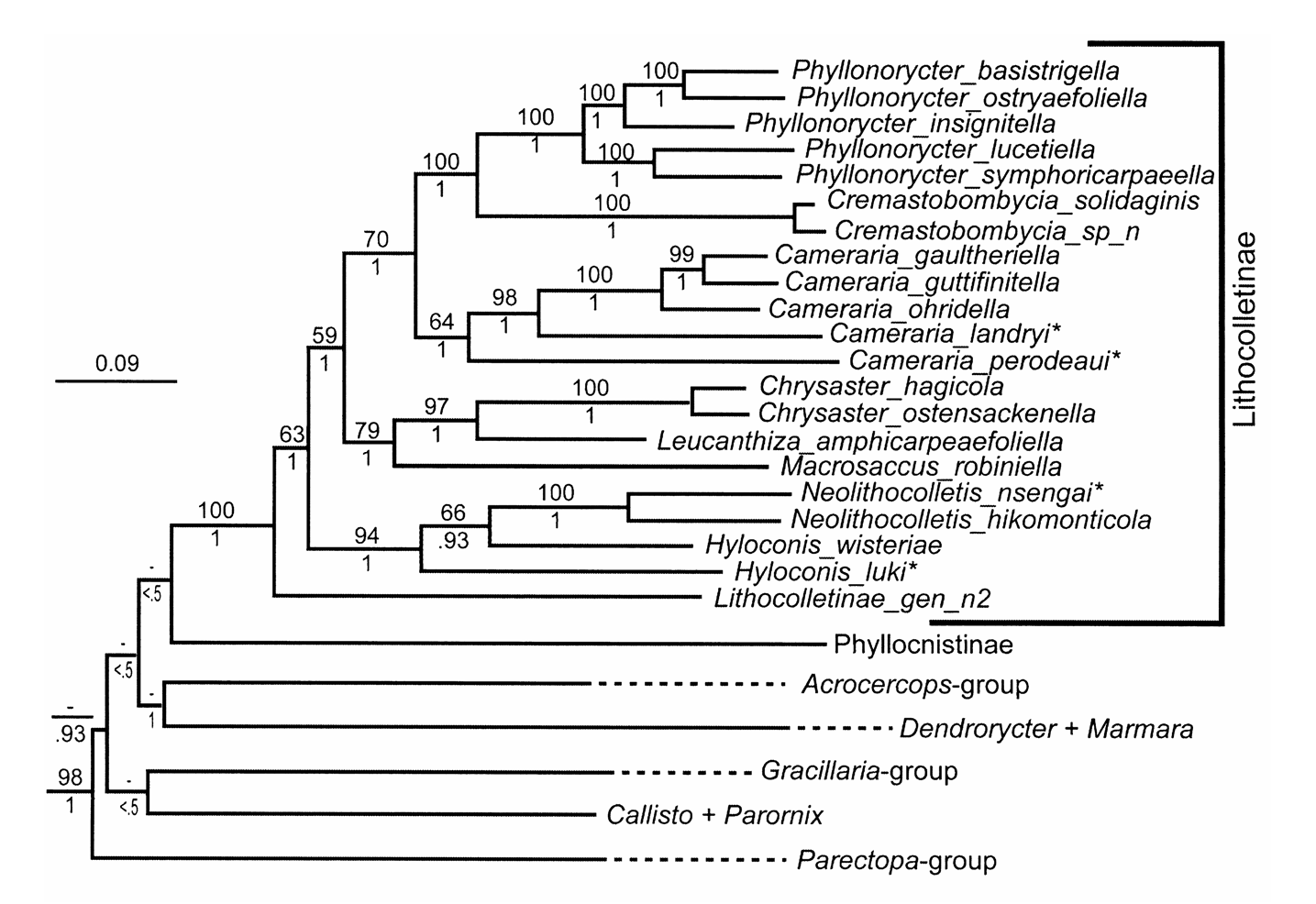
Diagnosis. Cameraria is morphologically very similar to Phyllonorycter in adult external features, particularly in wing venation (except C. fasciata ) and general external structure ( Kumata 1963). Vertex tufted like in most Lithocolletinae species but different from Chrysaster , Protolithocolletis , and Leucanthiza where vertex is smooth. The rough appearance of vertex is due to the neck plumes which are long and project forwards above the vertex between antennae ( Vári 1961). Cameraria differs from Phyllonorycter by the usually apically black margined white markings of forewing (except C. fasciata ), a character shared with Cremastobombycia . Wing venation is identical to Phyllonorycter in having 5 apical veins in forewing. Hindwing venation is similar to Phyllonorycter , and Chrysaster in the absence of vein M 2. Sternum VIII in males forms a characteristic flap, often bifid caudally, laying under valvae like in many Lithocolletinae genera except Chrysaster , Leucanthiza , Macrosaccus and Protolithocolletis . The conspicuous adult characters separating Cameraria from many other closely related genera are found in the apical part of tegumen in male genitalia. It can be distinguished from Phyllonorycter by the presence of a pair of setae on the apex of tegumen, which are absent in Phyllonorycter . The apex of the tegumen in Cameraria may bear appendages. A pair of apical setae present in Cameraria is also present in Chrysaster , Macrosaccus , and Porphyrosela and may represent an apomorphy uniting these genera. However, many other morphological characters of adults including wing venation and male/female genital morphology inhibit making a confusion of Cameraria with these genera. Cameraria was described based on larval morphology ( Chapman 1902), and the larval characters still remain the most significant diagnostic characters of this genus ( Kumata 1993). Cameraria makes rather flat upperside mines in contrast with the tentiform mines of Phyllonorycter . Larvae of Cameraria are hypermethamorphic, where the body form remains flattened throughout most of larval life, only to become cylindrical in the final instar ( Chapman 1902; Braun 1914; Opler & Davis 1981). On the contrary, larvae of Phyllonorycter are flat for only the first three sap feeding instars, and the following tissue feeding instars are cylindrical. Abdomen of the last instar of Cameraria have a series of sclerotized shields both on dorsal and ventral surfaces, prominently different from Phyllonorycter . In body chaetotaxy, the lateral group is bisetose on body segments (except on the segments IX and X) and the subventral group bisetose (SV1 and SV2) on ventral prolegs. Thoracic legs in Cameraria reduced to ventral protuberances (except in C. hikosanensis ), but in tropical Cameraria they are well-developed ( Kumata 1993). According to Kumata (1993) there are other slight morphological differences separating tropical Cameraria species from Holarctic congeners, such as the three subventral setae on the ventral prolegs (except in C. bauhiniae ), and the complete transtilla in male genitalia (in virgulata species group). Pupa without cremaster in Cameraria but with cremaster in Phyllonorycter . Pupation occurs under a flat circular cocoon within the mine, the character also differentiating Cameraria from the other genera of Lithocolletinae . Exuvium protrudes from mine as in Phyllonorycter . The vast majority of Cameraria species are restricted to a single host ( Opler & Davis 1981; De Prins & De Prins 2012). Cameraria and other Lithocolletinae mostly utilize the same host plant families, except Annonaceae , Hippocastanaceae and Lauraceae . No other lithocolletine genera except Cameraria , are recorded feeding on these latter plant families ( De Prins & De Prins 2012). Cameraria also differs from Phyllonorycter in COI sequence data. These two groups are separated by at least 10% sequence divergence for the taxa that were sampled in this study ( Fig. 3 View FIGURE 3 ).
Diagnosis of Afrotropical Cameraria . Afrotropical Cameraria are generally small moths, having a wing length of 1.7–2.9 mm. In the Afrotropical region there are eight Cameraria species in five species groups: 1) hexalobina species group; 2) landryi species group; 3) sokoke group; 4) perodeaui group, and 5) torridella species group. The species within the sokoke and landryi groups are differ greatly in their genital morphology, but are nearly indistinguishable in their external habitus. We tentatively place these two species into two informal species groups for convenience in their taxonomic treatment until additional data can clarify their phylogenetic relationships. Some Afrotropical Cameraria species such as C. hexalobina ( Vári, 1961) are highly distinctive externally due to the absence of white markings on forewings, or there might be just traces of them.
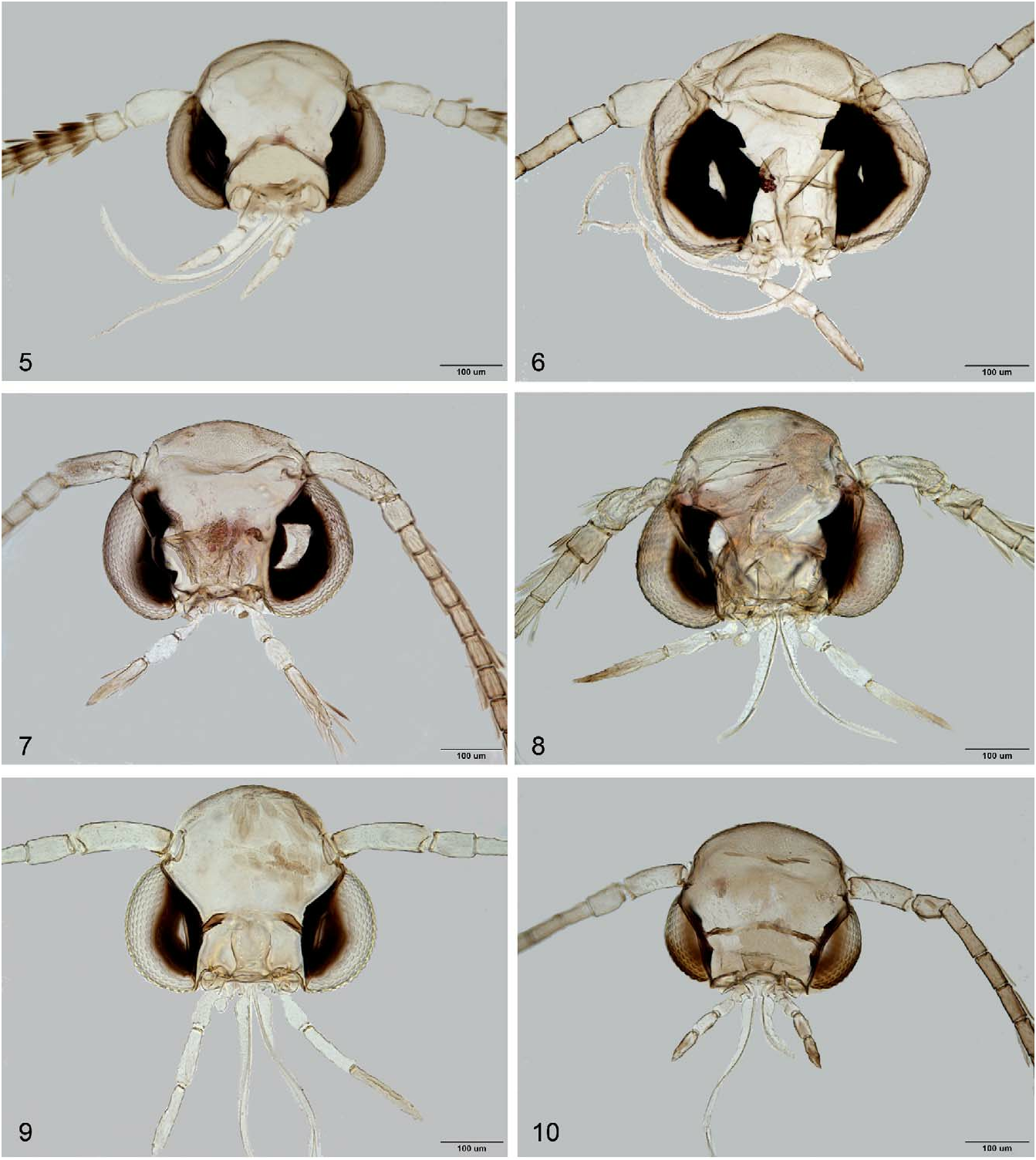
Head: Vertex plate jointed with frontoclypeus, head tufted with erect long piliform scales, whitish or light ochreous; frons covered with appressed smooth scales, shiny white or golden; eyes large, ocular index ca. 0.6–0.8, interocular index ca. 1.2–1.4 Antenna from ca. as long as forewing to ca. 20% shorter, smooth scaled, filiform; scape short thickened, bearing pecten of different length. Proboscis developed, naked, of medium length, ca. 2.0–2.5× length of labial palpus. Maxillary palpus small, rudimental, bi-segmented, apical maxillary palpomere almost globular. Labial palpus moderate, porrect, filiform, drooping, straight, with ratio of segments from base 0.5–1.0:1:1.25–2.2 ( Fig. 6 View FIGURES 5–10 ).
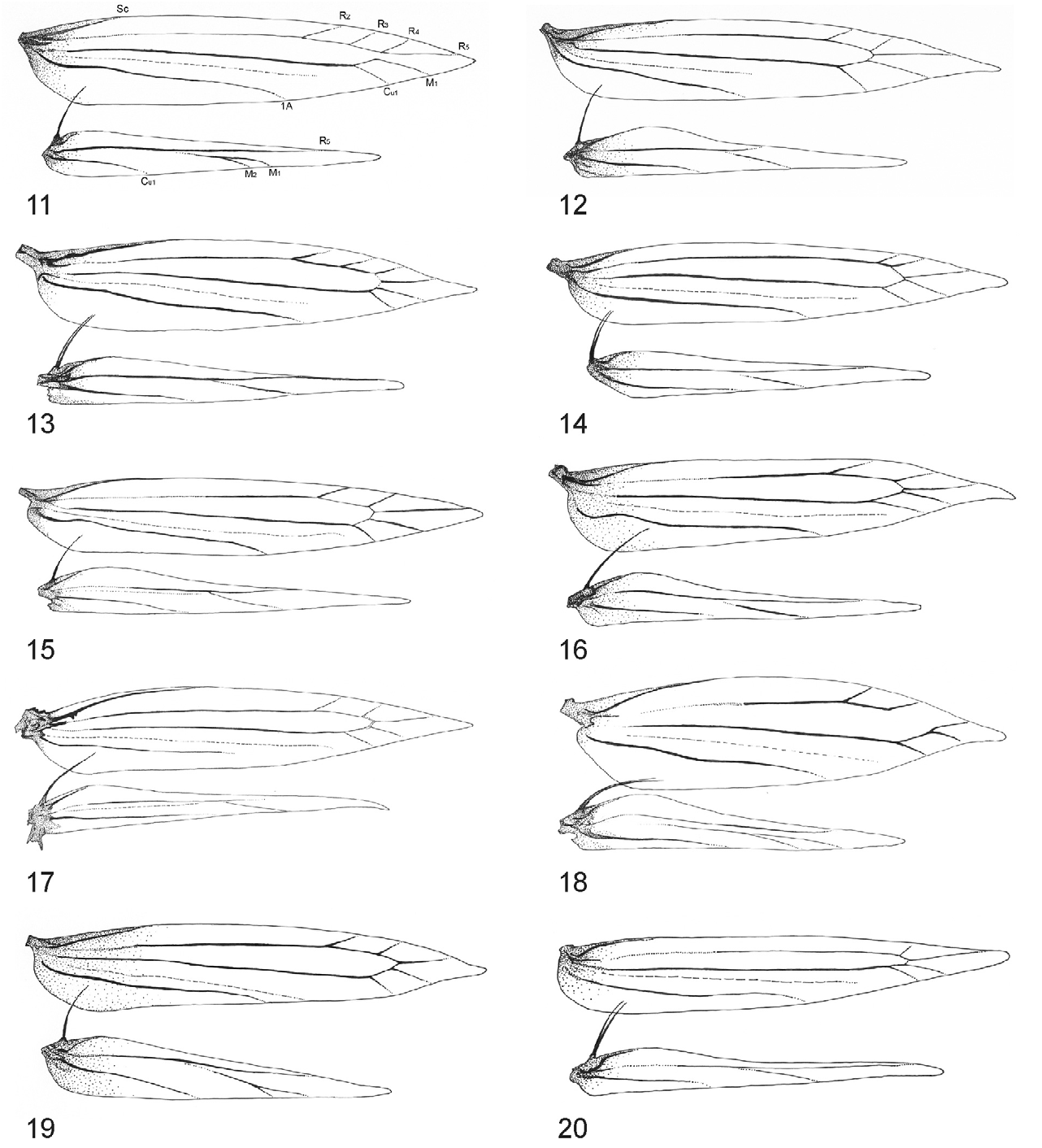
Thorax: Forewing ground colour orange-ochreous or beige-ochreous or dark brown-ochreous with white and black or only black markings; white markings are edged apically; fringe long, particularly near tornus and dorsum, reaching width of wing in forewing and ca. 3× width of wing in hindwing. Descaled forewing lanceolate, slender, and pointed. Venation with 8 veins, apical part with 5 veins R 3, R 4, R 5, M 1, Cu 1; M 1 and Cu 1 separate, CuP indistinct (fold) over entire length, 1A strong, separate. Hindwing lanceolate, maximum width/hind wing length is 0.13, venation reduced 4 veined: Sc very short terminating near base of costa, Rs very long, extedning almost to apex of hindwing, M 1 single branched, basal 2/3 of M 1 indistinct, parallel to Rs, distal part of M 1 extending along dorsal margin, Cu 1 strong, ending slightly before 1/2 of dorsum; A 1 vestigial ( Figs 14, 15 View FIGURES 11–20 ). Frenulum in male a single stout bristle, frenula in female 2 tightly appressed bristles. Legs slender, with darker rings; epiphysis on foreleg absent, mid-tibia bearing a pair of tibial spurs; hind tibia thickened, with long fine loose hairs, long medial and short apical spurs, hind tarsus smooth, slender, ca. 1.1–1.3× as long as tibia.
Abdomen. Margins of abdomen opening narrowly sclerotized slightly broader towards S2, the sclerotized margination of abdomen opening well connected on T2 and unconnected on S2; S2 apodemes of median length, ending just beyond the opening, slender, with barbed bases, slender distally, a pair of tiny spicules present on each abdominal sternum sublatero-anteriorly. Sternum VIII in adult males well developed, flap-like, extended, in many species bifurcate caudally.
Male genitalia. Tegumen rather long, with a pair of setae on conical apex. Valvae symmetrical, slender, long, narrow, weakly curved, gently attenuated, or enlarged at cucullus area, haired and /or covered with tubercles. Transtilla incomplete in majority of species, however complete in C. varii and C. torridella . Vinculum well developed, V- or W-shaped (in C. varii ) with prominent saccus or with long vincular ventral appendage ( C. zaira ). Anellus well developed, strongly sclerotized, tubular ( C. hexalobina and C. landryi ) or weakly developed ( C. varii ), can carry well developed fultura superior ( C. zaira ). Aedoeagus relatively simple, with enlarged coecum, gradually tapering towards slender apex; cornuti present or absent, some exogenous folds, invaginations might be present as well.
Female genitalia. Papillae anales flat caudally, fused. Segment VIII short, weakly connected to segment VII. Posterior apophyses without enlarged bases, slender; anterior apophyses originating at middle of segment VIII, slender or absent ( C. varii ). Segment VIII in most Afrotropical Cameraria species well connected to segment VII except C. perodeaui , where segment VIII retains only week lateral connections with segment VII. Ostium bursae opens at the posterior margin of segment VII near the connection with segment VIII either at depth of trapeziform sclerotized posterior extention of segment VII, or at emargination of posterior margin of segment VII, except C. torridella (where ostium bursae opens in median part of segment VII) and the perodeaui group (where ostium bursae opens in subposterior part of segment VII). Ductus bursae long, with triangular sclerotized plate crossing ductus bursae at subproximal part, near antrum (except C. torridella , C. landryi , and C. perodeaui ); antrum slightly wider and melanized or very broad sac-shaped ( C. varii ). Corpus bursae oval, conspicuous distinct from ductus bursae, elongate sac-shaped, or with slight dilation of ductus bursae ( C. perodeaui ), with one or two signa areas or without signum ( C. perodeaui ). One signum usually crossed by fine median ridge.
Biology. In most cases a large, oblong, or tentiform mine on upperside of leaf, with one fold; no loose frass; pupation in a disc, in a very flat, oval white cocoon which is constructed on lower surface of mine; pupa protrudes through upper epidermis before adult emerges ( Vari 1961: 211–212). However, it might construct a tentiform underside mine ( torridella group).
Distribution. Afrotropical Cameraria occur in primary rain forests and/or mixed forest/savannah biotopes.
Relationships to other genera. Cameraria appears ancestral to a clade that constitutes Cremastobombycia + Phyllonorycter (BP = 70%, PP = 1.0; Fig. 4 View FIGURE 4 ). Although our taxon sampling of Cameraria was limited to five species, those taxa formed a weakly supported monophyletic group (BP = 64%; Fig. 4 View FIGURE 4 ). The two African species sampled in this study are ancestral to those from Europe ( C. ohridella ) and North America ( C. gaultheriella , C. guttifinitella ). These three genera share a modified male eighth abdominal sternite, extending caudally to form a flap under the valvae; and forewing veins R3, R4, and R5 extending to the costa. The valvae of Cameraria are symmetrical, slender, long, narrow, usually haired, but the morphology of the valva is highly variable in Cremastobombycia and Phyllonorycter .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Cameraria Chapman, 1902
| Prins, Jurate De & Kawahara, Akito Y. 2012 |
Cameraria
| Chapman, T. A. 1902: 141 |