Lagothrix flavicauda (Humboldt, 1812)
|
publication ID |
https://doi.org/10.1093/mspecies/seab013 |
|
publication LSID |
lsid:zoobank.org:pub:5F48507B-673C-475A-846E-7642A021F471 |
|
persistent identifier |
https://treatment.plazi.org/id/03B03D78-FFF9-FF87-FF38-608291C0F945 |
|
treatment provided by |
Felipe |
|
scientific name |
Lagothrix flavicauda (Humboldt, 1812) |
| status |
|
Lagothrix flavicauda (Humboldt, 1812) View in CoL
Yellow-tailed Woolly Monkey
Simia flavicauda Humboldt, 1812a:343 View in CoL . Type locality “Les rives de l’Amazone dans les provinces de Jaen et de Maynas,” Peru.
Simia [( Stentor View in CoL ?)] flavicauda: Humboldt, 1812b:355 View in CoL . Type locality “province de Jaen,” Cajamarca, Peru; restricted to Puca Tambo, San Martín, upper Rio Mayo, about 50 miles (80 kilometers) east of Chachapoyas, 5,100 ft (1,554 meters) above sea level by Fooden (1963:242; see “Nomenclatural Notes”).
Stentor flavicaudatus: É. Geoffroy Saint-Hilaire, 1812:108 . Unjustified emendation of flavicauda Humboldt (1812a) View in CoL ; based on Humboldt’s (1812a:343) Simia flavicauda View in CoL .
Cebus flavicaudatus: Desmarest, 1816:341 . Name combination; based on Humboldt’s (1812a:343) Simia flavicauda View in CoL .
Mycetes flavicaudatus: Kuhl, 1820:30 . Name combination; based on Humboldt’s (1812a:343) Simia flavicauda View in CoL .
“Rothe Choro” Poeppig, 1832:col.100. Type localityYurimaguas (Province of Alto Amazonas, Department of Loreto, Peru); vernacular, unavailable name.
M [ ycetes]. caraya: Wagner, 1840:182 , supplement: abt. I. Part; not caraya Humboldt, 1812 ; based on Humboldt’s (1812a:343) Simia flavicauda .
M [ ycetes]. ursinus: I. Geoffroy Saint-Hilaire, 1851:53 . Part; not ursinus É. Geoffroy Saint-Hilaire (1812) ; a “variété”; based on Humboldt’s (1812a:343) Simia flavicauda .
Mycetes flavicauda: Schlegel, 1876:147 . Name combination; based on Humboldt’s (1812a:343) Simia flavicauda View in CoL .
Alouatta ursina: Forbes, 1896:198 . Not ursina Humboldt, 1812 .
Lagothrix ( Oreonax) hendeei Thomas, 1927a:156 View in CoL . Type locality “Puca Tambo, about 50 miles (80 kilometers) east of Chachapoyas, N. Peru, 5,100 ft (1,554 meters), Peru.”
Oreonax hendeei: Thomas, 1927c:596 . Name combination.
[ Alouatta View in CoL ] flavicauda: Tate, 1939:217 View in CoL . Name combination; based on Humboldt’s (1812a:343) Simia flavicauda View in CoL .
[ Lagothrix View in CoL ] hendeei: Tate, 1939:214 . Name combination; based on Thomas’ (1927a:156) Lagothrix ( Oreonax) hendeei View in CoL .
Lagothrix flavicauda: Fooden, 1963:241 View in CoL . First use of current name combination. Based on Humboldt’s (1812a:343) Simia flavicauda View in CoL and Thomas’ (1927a:156) Lagothrix ( Oreonax) hendeei View in CoL .
Oreonax flavicauda: Groves, 2001:195 View in CoL . Name combination.
CONTEXT AND CONTENT. Context as for genus. No subspecies of Lagothrix flavicauda View in CoL are recognized ( Fooden 1963; RuizGarcía et al. 2014; Di Fiore et al. 2015).
NOMENCLATURAL NOTES. The taxonomic identity of Lagothrix flavicauda remained uncertain for more than 150 years since Humboldt’s (1812a) original description of Simia flavicauda . This uncertainty was due to the lack of type material and because Humboldt (1812 a, 1812b) considered S. flavicauda a species of howler monkey ( Fooden 1963; Serrano-Villavicencio and Silveira 2019), a presumption maintained by subsequent authors: Kuhl (1820), Tschudi (1844), I. Geoffroy Saint-Hilaire (1851), Forbes (1896), and Elliot (1913). Thomas (1927a) described a new species of woolly monkey, Lagothrix ( Oreonax) hendeei , based on material collected by R. W. Hendee at Puca Tambo. After comparing this new species with Humboldt’s original description of S. flavicauda, Thomas (1927b:363) stated that the later species was probably “a local Lagothrix , perhaps L. lagotricha . ” However, Thomas (1927c) reconsidered, concluding that morphological differences were sufficient to place it in its own genus, Oreonax . Fooden (1963) in the first comprehensive revision of Lagothrix determined that Humboldt’s (1812 a, 1812b) Simia flavicauda was not a howler monkey, but the woolly monkey Oreonax hendeei of Thomas (1927c), and renamed it Lagothrix flavicauda .
Fooden (1963) redefined the type locality of L. flavicauda as Puca Tambo, department of San Martín, but there is confusion as to where Puca Tambo is located. Stephens and Traylor (1983) gave the coordinates as 06°10′S, 77°16′W which, falling in what is now the department of Amazonas, seemed to be incorrect. They cited Vaurie (1972), who gave the coordinates as 06°09′S, 77°11′W, which are in San Martín.Arroyo-Cabrales (2008) reviewed information given by various authors, but was unable to determine the exact location of Puca Tambo. Confusingly, in the gazetteer presented by Arroyo-Cabrales (2008), Puca Tambo is in the department of San Martín, but the coordinates he gave placed it in Amazonas. This locality is evidently near the border of Amazonas and San Martín, but we have no exact location. Judging by the elevation given by Thomas, 1,554 m, it is most likely that the type material of Lagothrix ( Oreonax) hendeei was collected in the department of Amazonas.
The phylogenetic position of L. flavicauda was first investigated by Groves (2001). Based on a parsimony analysis of 20 cranial features, Groves (2001) obtained a poorly supported clade, containing L. flavicauda and an unknown species of Ateles , and no other species of Lagothrix ( Matthews and Rosenberger 2008; Rosenberger and Matthews 2008), placing flavicauda in the monotypic genus Oreonax , as proposed by Thomas (1927c). Matthews and Rosenberger (2008) and Rosenberger and Matthews (2008) found that Groves’ (2001) results were from a specific combination of taxa and characters and were not representative of a strong phylogenetic signal. They also indicated the absence of a stable, replicable cladistic result and proposed the rejection of Oreonax as a valid genus. The results obtained by Groves (2001) were refuted by the morphology-based phylogenies presented by Paredes-Esquivel (2003) and Serrano-Villavicencio (2016). Molecular phylogenies presented by Ruiz-García et al. (2014) and Di Fiore et al. (2015) also supported the monophyly of Lagothrix , including flavicauda .
DIAGNOSIS
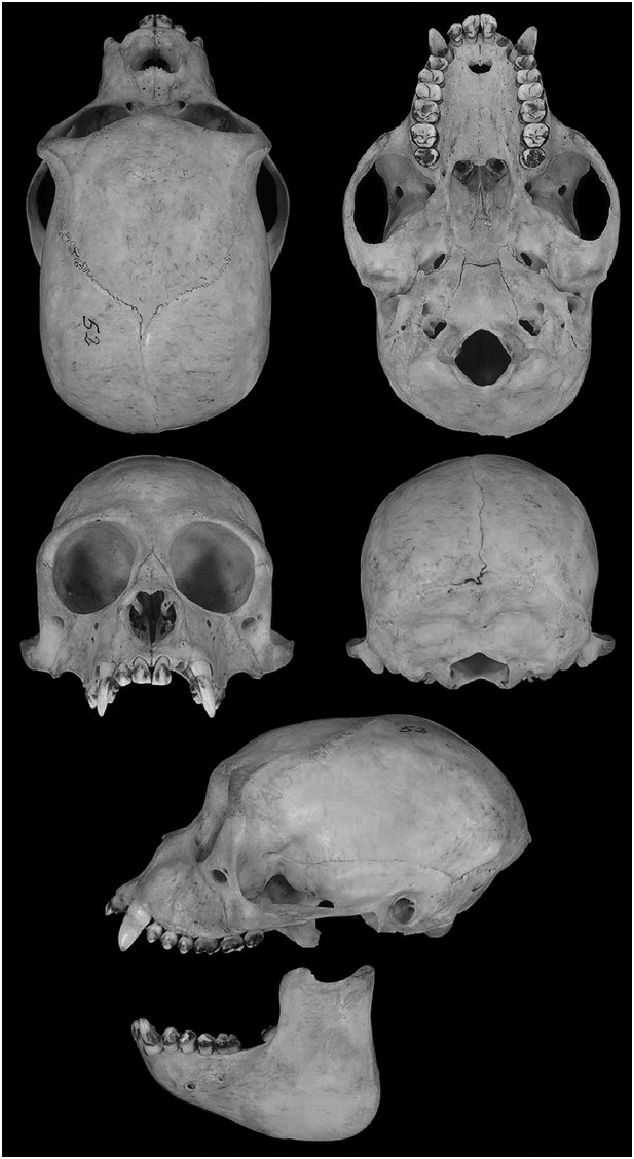
The fur of Lagothrix flavicauda is longer and thicker than in L. lagothricha (the common woolly monkey— Mittermeier et al. 1977). The face is pigmented dark gray with a well-defined white circumbuccal patch and white supraorbital hairs that can be poorly or well-defined. Lagothrix flavicauda has conspicuous golden-yellow hairs in the genital area and distal one-half of the ventral surface, surrounding the caudal pad of the tail, which is absent in L. lagothricha ( Fig. 1 View Fig ). This genital tuft is abundant and dense in males, less abundant, and slightly separated into discrete segments in females ( Fig. 2 View Fig ; Fooden 1963; de Macedo-Ruiz and Mittermeier 1979; Groves 2001; Cornejo 2013; Serrano-Villavicencio 2014). Compared to L. lagothricha , L. flavicauda has a narrow braincase, wider zygomatic arches, and more prominent medial superciliary protuberances ( Fig. 3 View Fig ; Fooden 1963).
GENERAL CHARACTERS
Lagothrix flavicauda is a large atelid, slightly larger than L. lagothricha , with adult male head–body length = 561.6 ± 77 mm SD and total length = 1,136.7 ± 66 mm SD ( n = 3); and adult female head–body length = 529 ± 28 mm SD and total length = 1,155 ± 27 mm SD ( n = 5— Serrano-Villavicencio 2014). Measurements (mm) for the holotype, Lagothrix ( Oreonax) hendeei ( Thomas 1927a:156) were: head–body length 520; tail length 560; length of hind foot 145; ear length 32.5. Leo Luna (1984) reported an average body mass of about 10 kg, and Cornejo (2013) reported a range of 8.3–10 kg for adult males and a value of 5.7 kg for female body mass. The fur of adult L. flavicauda is deep mahogany for both males and females, which at first appears to be uniform. However, color intensity varies across the body. The pelage of the head, crown, and upper parts of the back is blackish with a brown nuance that gradually increases toward the back to change to a lighter reddish-brown, and this color continues to the end of the tail ( Fig. 2 View Fig ; Thomas 1927a, 1927b; Fooden 1963; Serrano-Villavicencio 2014).
The pelage of L. flavicauda is characteristically dense, soft, and particularly long on the chest. Measurements of the hairs in the cephalic region have an average length of 5 mm. Hair length increases on the neck to 25 mm, and 30 mm in the dorsal region. The forelimb and hindlimb pelage are of similar length ( 15 mm) but may be slightly longer on the forelimbs ( 20 mm). These measurements were taken from a specimen in the Universidad Nacional Mayor de San Marcos, (MUSM [Museo de Historia Natural] 23155). Ears are partially hidden, with the distal portion of the helix more evident. The ears have short hairs that are lighter than in the cephalic region and lack tufts. The ventral distal portion of the tail has a dermatoglyphic pad, which is devoid of hair and surrounded by bright yellow pelage. The genital region has a yellow tuft of the same color as those surrounding the caudal pad on the underside of the tail.
The braincase is narrow, zygomatic arches widely flaring, and temporal fossa short and wide; orbital margin enters almost at a right angle in the intersection with the zygomatic bone; squamosal process of zygoma twice as deep as temporal; medial superciliary protuberances prominent; a large foramen is present behind M3 at the root of the pterygoid ( Fig. 3 View Fig ). The bullae are low, flat, and descending a little below the basioccipital ( Tate 1939; Fooden 1963). The nasals are not definitively concave or greatly upturned at their tips, and the incisors are distinctly prognathous ( Thomas 1927a; Hill 1960). Mean (± SD) skull measurements (mm) for adult L. flavicauda (males and females, respectively; n in parentheses) as calculated from values given in Thomas (1927a), Hill (1960), Fooden (1963), and Serrano-Villavicencio (2014) were: greatest cranial length, 104.1 ± 2.6 (3), 106.5 ± 2.2 (2); bizygomatic breadth, 70.9 ± 3 (3), 73 ± 1.2 (2); braincase width, 55.9 ± 1.9 (3), 57.4 ± 3.7 (2); postcanine length, 26.7 ± 1.2 (2), 28.2 (1).
The inner margins of the rows of the upper molariform teeth are more-or-less parallel and may become slightly oblique. The crown size of the upper postcanine dentition, from largest to smallest, is M1 or M2, M3, PM4, PM3, PM2. The lower postcanine dentition size series is m2, m1 or m3, pm2 or pm4, pm3. The molars are quadritubercular, each upper molar is wider than long, with low cusps and reduced ridges and bilophodont crowns. The base of the canine is robust and presents a triangular outline with an upper precanine diastema about one-half the length of the I2 crown. The incisors are long and moderately broad, the I2 is spatulate, slightly smaller than I1, and located just behind it. The posterior cingulum of the upper incisors is welldeveloped with an incipient lingual tubercle. The I1 possesses an almost straight cutting edge with the lingual tubercle more developed than in the lateral incisors. The I2 is slightly narrower than I1 ( Serrano-Villavicencio 2014).
DISTRIBUTION
Lagothrix flavicauda is endemic to central and northern Peru, along the eastern side of the Andean cordillera ( Fig. 4 View Fig ; Leo Luna 1980; S. Shanee 2011; Aquino et al. 2016, 2017; McHugh et al. 2019) mostly at elevations between about 1,400 and 2,800 m (S. Shanee 2011; Aquino et al. 2016, 2017). The majority of the records are in the departments of Amazonas and San Martín (S. Shanee 2011), but L. flavicauda has also been recorded in the departments of Huánuco (S. Shanee 2011; Aquino et al. 2016), La Libertad ( Parker and Barkley 1981), and Loreto ( Patterson and López Wong 2014). At two localities, it has been recorded at lower elevations of just over 1,000 m ( Allgas et al. 2014; Patterson and López Wong 2014). A new population was discovered recently much further south (~ 200 km), in Junín department, at elevations between 1,400 and 1,750 m ( McHugh et al. 2019). In Amazonas, L. flavicauda has been recorded at many localities since its rediscovery in the 1970s ( Mittermeier et al. 1975). Its distribution here is limited in the north and northwest by the lowlands of the Marañon River and in the west by the Utcubamba River ( Leo Luna 1980; S. Shanee 2011). Small relict populations have been recorded to the west of the Utcubamba River (S. Shanee 2011). In San Martín, L. flavicauda is present in the highlands on both sides of the Mayo River valley and to the west along the border with Amazonas and La Libertad, all the way south to the border with Huánuco department (S. Shanee 2011; Allgas et al. 2014). In La Libertad, it has been confirmed in the extreme eastern province of Pataz ( Parker and Barkley 1981; S. Shanee 2011). In Huánuco, its distribution extends from the border of San Martín (S. Shanee 2011; Aquino et al. 2016, 2017), south along the left bank of the Alto Rio Huallaga toward Pasco ( Aquino et al. 2016, 2017). In Loreto, a small population has been recorded in the montane forests in the far southwest of the department, in the provinces of Alto Amazonas, and possibly also Datem del Marañón, on the border of the Cordillera Escalera in San Martín ( Patterson and López Wong 2014). In 2019, a chance encounter during biological surveys led to the discovery of a population of L. flavicauda in the department of Junín in the Inchatoshi Kametsha Conservation Concession, near Río Pampa Hermosa ( McHugh et al. 2019), far to the south of the rest of the species’ distribution. So far, no records of L. flavicauda exist for the department of Pasco, between the southernmost part of its distribution in Huánuco and the Junín population ( Aquino et al. 2019). Lagothrix flavicauda is thought to be absent from areas of suitable habitat in Ucayali ( Fig. 4 View Fig ; Aquino et al. 2019). There are no known fossils that can be attributed to Lagothrix .
No studies of locomotion and posture have been conducted with L. flavicauda , but as with other Lagothrix taxa, it is a primarily arboreal quadruped and climber species ( Fleagle 2013). Lagothrix lagothricha travels quadrupedally (41.8% of bouts), uses suspensory locomotion (8.6%), climbs (38.8%), and travels and leaps (10.8%— Defler 1999). During feeding L. lagothricha is quadrupedal (42.8% of bouts), uses suspensory locomotion (7%), climbs (44.2%), and leaps (6%— Defler 1999). Both L. flavicauda and L. lagothricha use their strong prehensile tails for support while moving, and L. lagotrhicha commonly hangs by the tail, with the hind legs resting on a vertical substrate, when feeding ( Defler 2010).
FORM AND FUNCTION
Lagothrix flavicauda has the typical atelid dental formula of i 2/2, c 1/1, p 3/3, m 3/3, total 36 ( Serrano-Villavicencio 2014). The vertebral formula of L. flavicauda is 7 C, 14 T, 6 L, 3 S with at least 14 caudal vertebrae, but the exact number is not known ( Serrano-Villavicencio 2014). Serrano-Villavicencio (2014) provided the following postcranial indices: intermembral, 95.08; humerofemoral, 95.04; brachial, 94.02; crural, 93.94.
ONTOGENY AND REPRODUCTION
As infants age, the facial pigmentation becomes darker in the maxillary region where the white hairs, diagnostic for Lagothrix flavicauda , are present. Pigmentation becomes darker in the nasal and interorbital regions, reaching a very dark brown to almost black in adults. The darkness does, however, decrease in intensity in the superolateral aspect of the orbits ( Serrano-Villavicencio 2014). Hill (1962) stated that one juvenile L. flavicauda collected by R. W. Hendee and held at the British Museum (Natural History), currently The Natural History Museum–London, has the coloration of an adult, but the pudendal tuft is not developed.
Aquino et al. (2015), observing L. flavicauda in Huánuco, suggested possible reproductive seasonality between January and April. During a 15-month study in La Esperanza, Amazonas, S. Shanee and N. Shanee (2011a) recorded just two copulations, both in January. No evidence was found during long-term studies at La Esperanza carried out between 2008 and 2019 (S. Shanee and N. Shanee 2011a; Fack et al. 2020a) to suggest reproductive seasonality. Leo Luna (1980) estimated only about one birth per year for every six adults. Between one and four infants per group, from a total of 13 groups, have been observed in Huánuco ( Aquino et al. 2015). Long-term observations on multiple groups in Amazonas documented a lot of within- and between-group variation in numbers of infants between years, between one and six, as young animals grow, and new infants are born (S. Shanee and N. Shanee 2011b; S. Shanee 2014b; Almeyda Zambrano et al. 2019; Fack et al. 2020b). No detailed reproductive or life history characteristics are available for L. flavicauda . In other Lagothrix , females emigrate at 6 (± 0.4 SD) years of age and female age at first reproduction is 9 (± 0.7 SD) years; males reach sexual maturity at 5.5–6 years; female cycle length is about 18 days; gestation length is about 210–225 days; and interbirth interval is 36.7 (± 4.7 SD) months ( Di Fiore et al. 2011).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Lagothrix flavicauda (Humboldt, 1812)
| Serrano-Villavicencio, José E, Shanee, Sam, Pacheco, Víctor, Cooper, Christine & Hamilton, Meredith J 2021 |
Oreonax flavicauda :
| Groves C. P. 2001: 195 |
Lagothrix flavicauda : Fooden, 1963:241
| Fooden J. 1963: 241 |
| Humboldt A. 1812: 343 |
Alouatta
| Tate G. H. H. 1939: 217 |
| Humboldt A. 1812: 343 |
Lagothrix
| Tate G. H. H. 1939: 214 |
Lagothrix ( Oreonax ) hendeei
| Thomas O. 1927: 156 |
Oreonax hendeei : Thomas, 1927c:596
| Thomas O. 1927: 596 |
Alouatta ursina :
| Forbes H. O. 1896: 198 |
Mycetes flavicauda :
| Schlegel H. 1876: 147 |
| Humboldt A. 1812: 343 |
Mycetes flavicaudatus :
| Kuhl H. 1820: 30 |
| Humboldt A. 1812: 343 |
Cebus flavicaudatus :
| Desmarest A. G. 1816: 341 |
| Humboldt A. 1812: 343 |
Simia flavicauda Humboldt, 1812a:343
| Humboldt A. 1812: 343 |
Simia
| Fooden J. 1963: 242 |
| Humboldt A. 1812: 355 |
Stentor flavicaudatus : É. Geoffroy Saint-Hilaire, 1812:108
| Geoffroy Saint-Hilaire E. 1812: 108 |
| Humboldt A. 1812: 343 |