Dysagrionidae, Cockerell
|
publication ID |
https://doi.org/10.11646/zootaxa.4934.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:79895443-4597-42A5-AF8A-023EACB20E10 |
|
DOI |
https://doi.org/10.5281/zenodo.4672711 |
|
persistent identifier |
https://treatment.plazi.org/id/03B487C2-0015-FF8D-FF5B-FA52FDAA16FC |
|
treatment provided by |
Plazi |
|
scientific name |
Dysagrionidae |
| status |
|
Reassessment of the Dysagrionidae
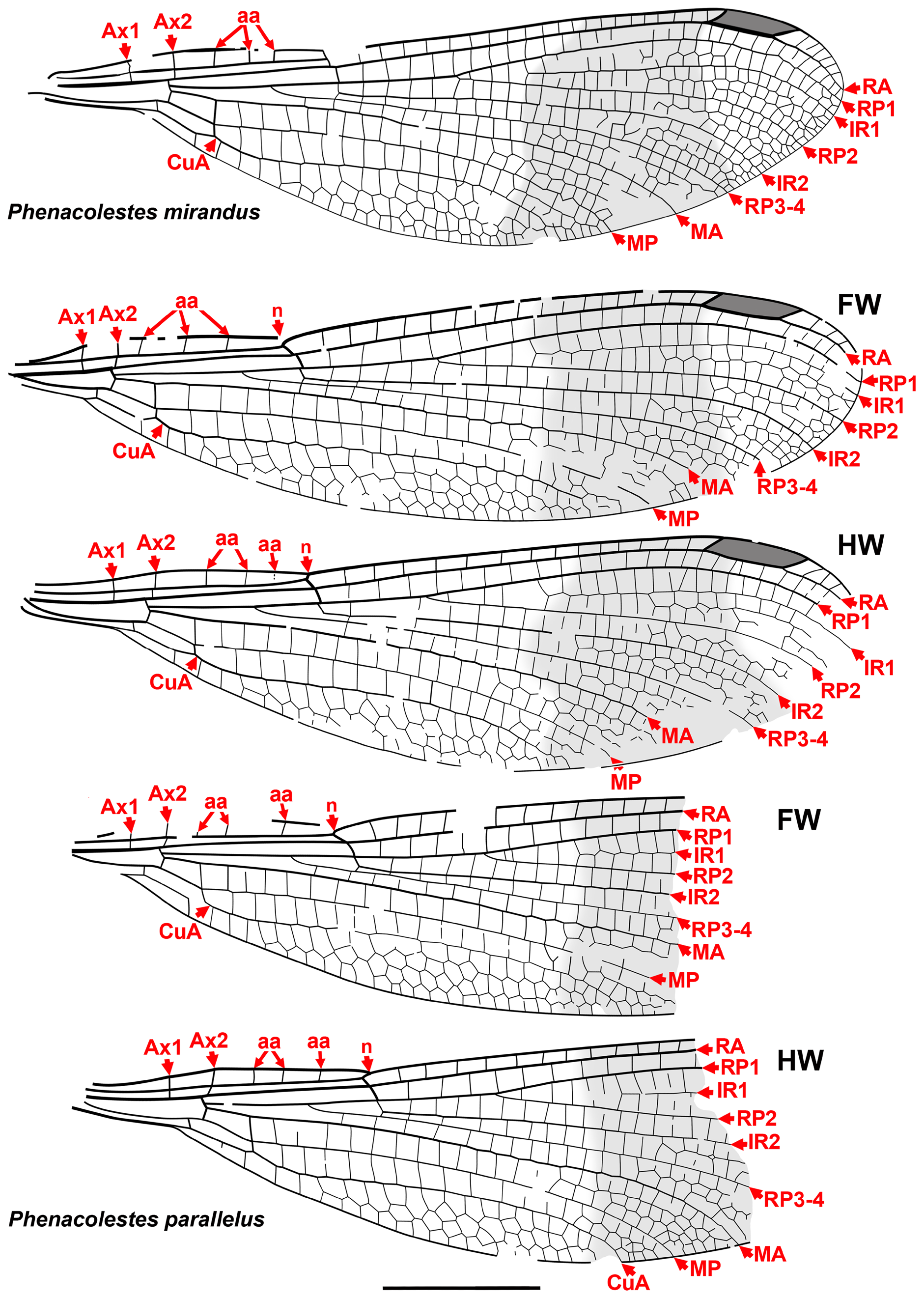
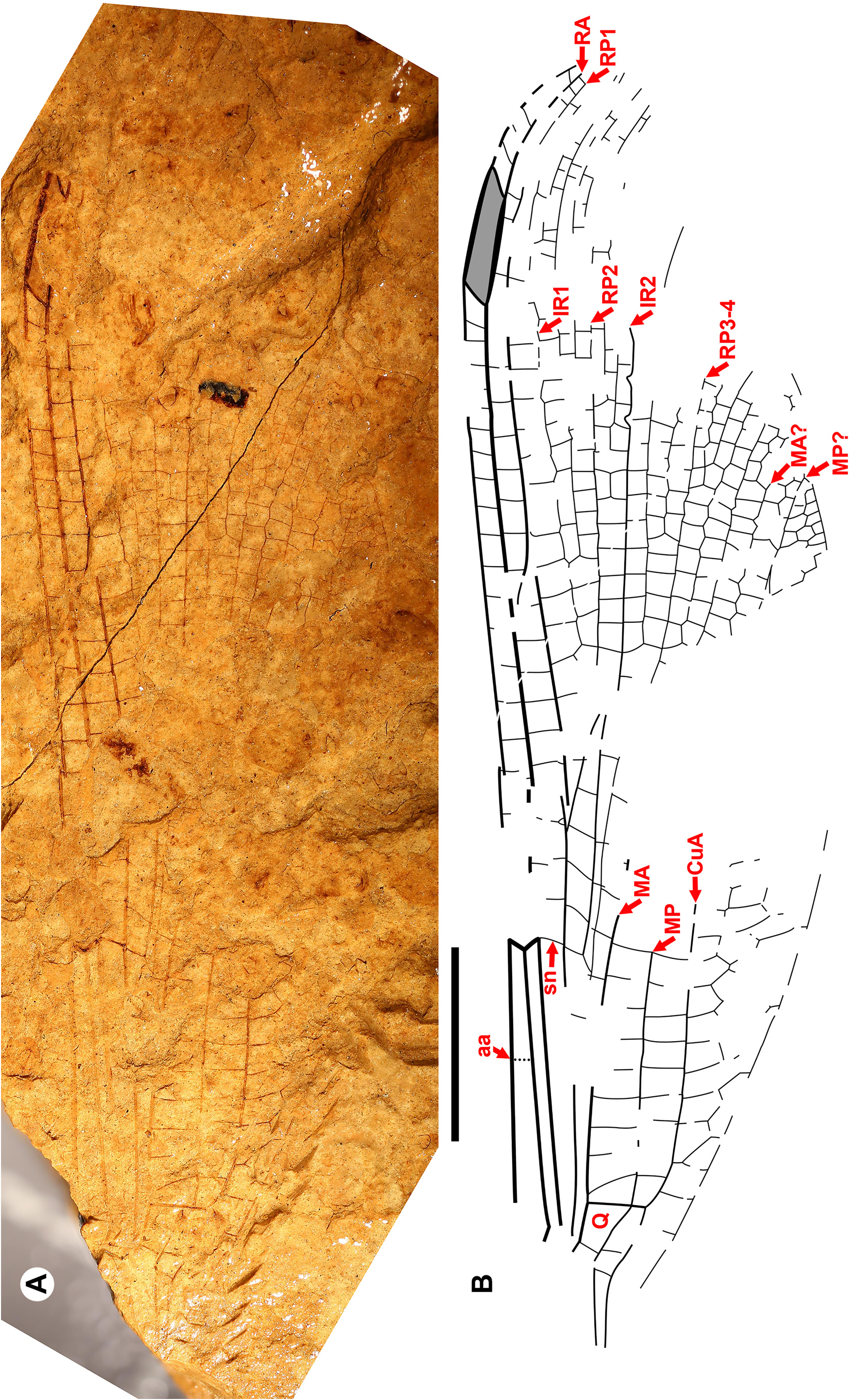
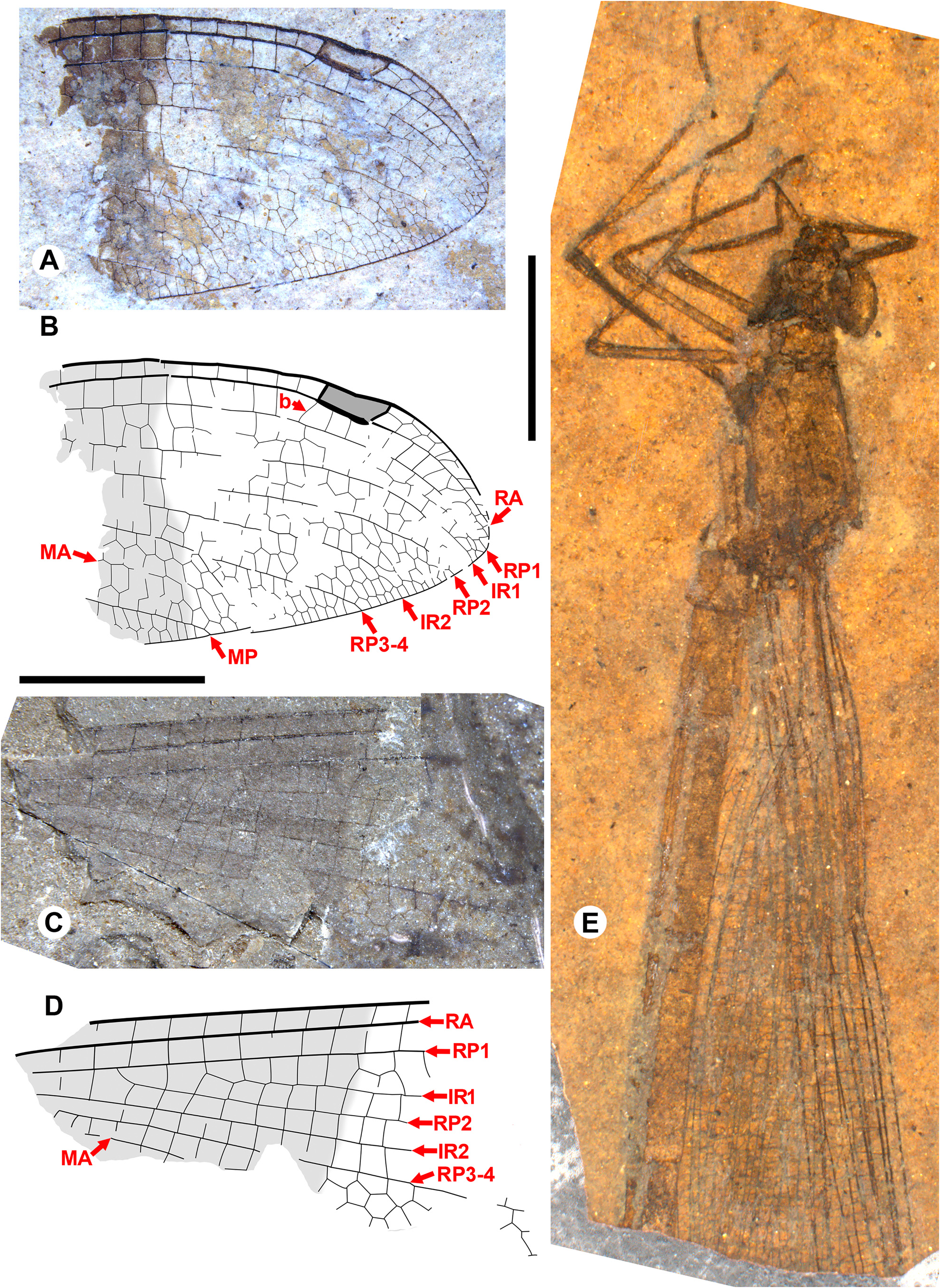
Taxon concepts based on the presence of accessory antenodal crossveins. The Dysagrioninae was originally proposed by Cockerell (1908a) as a subfamily of Agrionidae (Zygoptera) , comprised of the two extinct genera Dysagrion and Phenacolestes . He distinguished it from other agrionids solely by the presence of accessory antenodal crossveins, i.e., in the costal space between Ax2 and the nodus: two in Dysagrion and three in Phenacolestes ( P. mirandus Cockerell from the Priabonian shale of Florissant, Colorado was then its only confirmed species, Fig. 2 View FIGURE 2 ).
Calvert (1913) examined six specimens of P. mirandus , five of which had the antenodal region preserved, and found that three of these had three accessory antenodal crossveins, and the other two had two. He agreed with Campion (1913) that this variability made Cockerell’s Dysagrioninae untenable. Campion thought that P. mirandus most closely resembles the extant Neotropical Thaumatoneura McLachlan , which also has a variable number of accessory antenodal crossveins. Tillyard & Fraser (1939), Fraser (1957) and Nel & Paicheler (1994) did not recognise Cockerell’s Dysagrioninae , placing Phenacolestes in the Amphipterygidae ( Tillyard & Fraser 1939) and Pseudolestidae (Thaumatoneurinae) ( Fraser 1957; Nel & Paicheler 1994). Modern photographs clearly confirm the presence of three accessory antenodal crossveins in the holotype of P. mirandus (US National Parks Service website, see our drawing from this photograph, Fig. 2 View FIGURE 2 ).
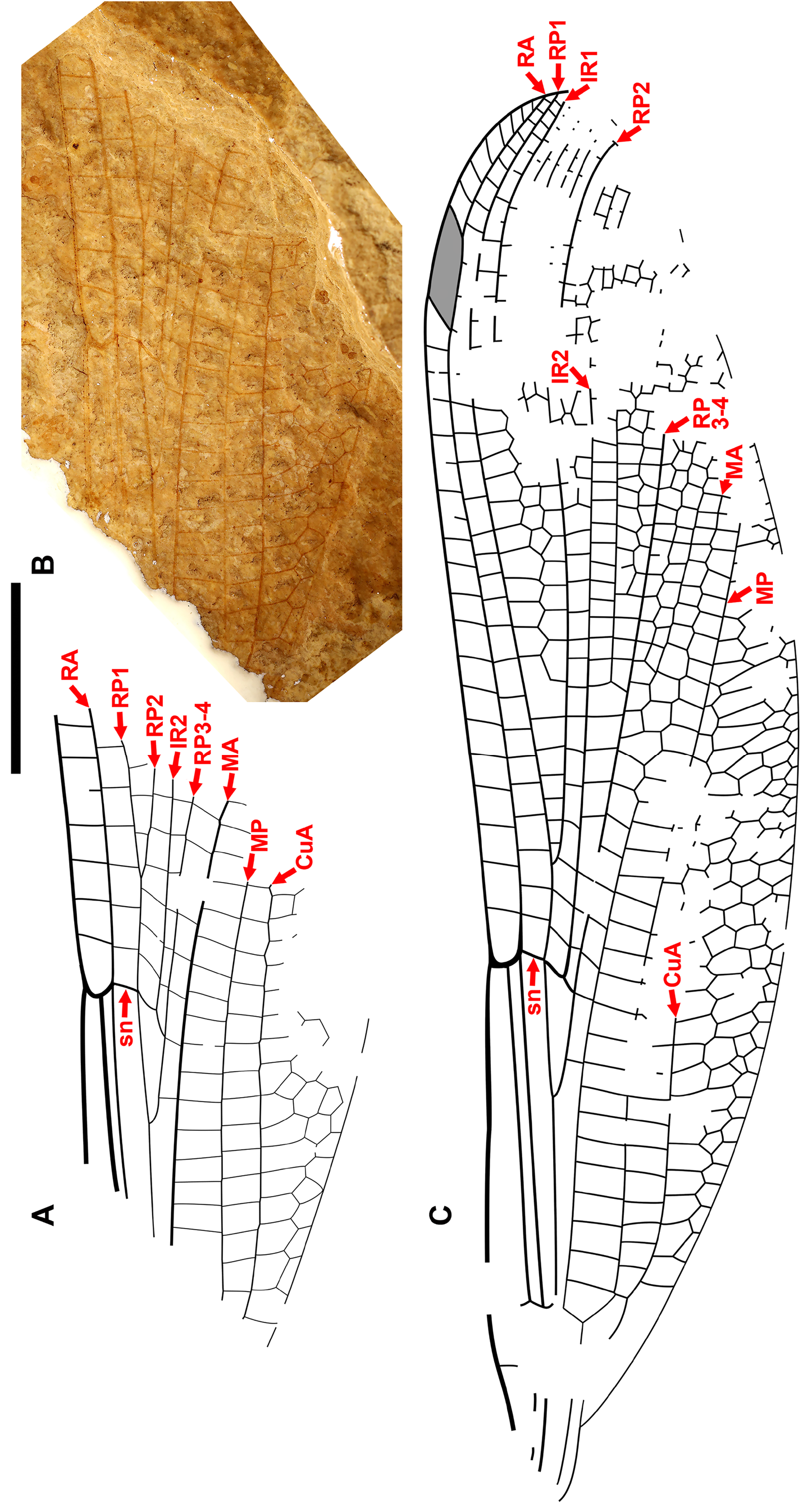
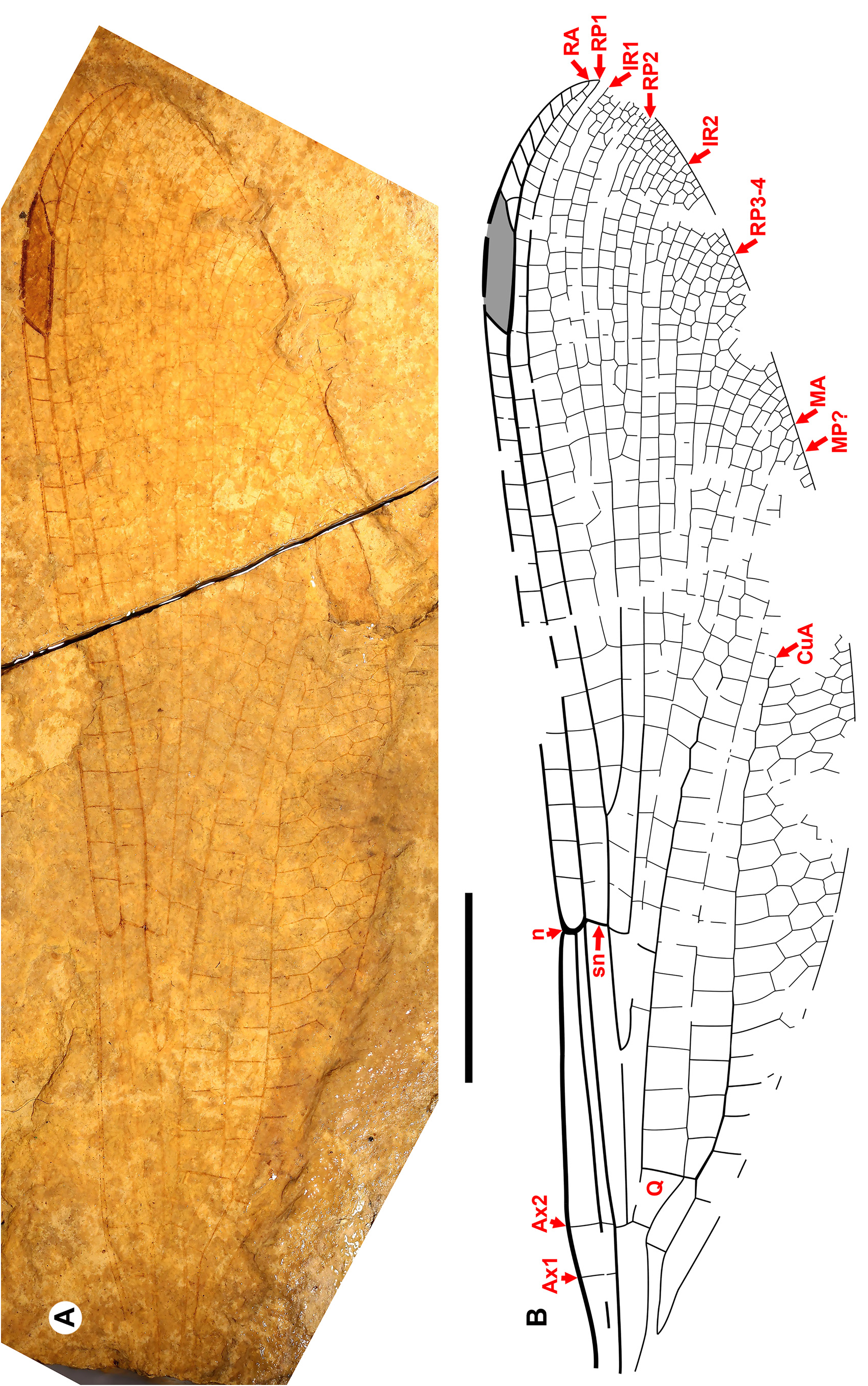
Cockerell (1908a) also described a new species from Florissant as Phenacolestes ? parallelus Cockerell , a tentative member of the genus as the region of the diagnostic accessory antenodal crossveins is not preserved (University of Colorado: UCM 4503, part and counterpart). Subsequently discovered specimens confirmed that the species is a Phenacolestes : a proximal half of a wing (Yale Peabody Museum: YPM IP 220974) has four accessory antenodal crossveins ( Cockerell 1908b) and a third, almost complete specimen (University of Colorado: UCM 4545), also from Florissant ( Cockerell 1908c), has three in both forewings and three in both hind wings. Here, we figure these ( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 ) as, to our knowledge, no specimen of the species has been previously illustrated other than by a poorquality photograph of UCM 4545 ( Cockerell 1908c, his Fig. 4 View FIGURE 4 ).
The third species attributed to the genus, P.? coloratus (Hagen) (mid-Miocene, Radoboj, Croatia) is poorly known, inadequately described, and we know of no published drawing or photograph ( Hagen 1848; and see Nel & Paicheler 1994; Nel et al. 2005b). We treat it as a possible member of the genus (adding the question mark), family, and suborder pending examination of the specimen.
The discussions of the above authors concerned the appropriateness of basing the taxon on a variable character state, i.e., two or three accessory antenodals in P. mirandus (the variability in P. parallelus described above was not known to them); the presence of two such crossveins in Dysagrion , however, was not doubted. Although Scudder’s original drawings should have established that this is not always the case, it remained unquestioned from the time of Cockerell’s works cited above to the present day. Scudder’s drawing of Dysagrion fredericii Scudder (Ypresian, Green River Formation, Wyoming) (1890: plate 6, Fig. 9 View FIGURE 9 ) type specimen 4167/4168 (now MCZ numbers 381A, B) shows two accessory antenodal crossveins; however, there are none in his drawing (1890: plate 6, Fig. 3 View FIGURE 3 ) of the type of D. packardii Scudder (Green River Formation, Wyoming) (Scudder cites the specimen as Packard number 146), a copy of which had already appeared in a work by Zittel (1885: Fig. 979) which Scudder edited. Tillyard & Fraser (1939: Fig. 3.1 View FIGURE 3 ) provided a drawing of the wing (re-drawn from Scudder?), adding two accessory antenodal crossveins, which Fraser subsequently reproduced (1957: Fig. 34.1 View FIGURE 34 ) (the specific epithet spelled “ packardi ” in both). Although Carpenter had the Museum of Comparative Zoology (MCZ) specimens available to him, his drawing of D. packardii (1992, his Fig. 52.1 View FIGURE 52 ) included these presumptive accessory antenodal crossveins, by which he in part distinguished the genus. Bechly (1996) based his tribe Congqingiini ( Congqingia Zhang and Petrolestes Cockerell ) on its members lacking accessory antenodal crossveins, in contrast to his Dysagrionini ( Dysagrion and Phenacolestes ), reported to possess them as above.
To resolve confusion as to the presence, and so the diagnostic usefulness, of accessory antenodal crossveins in Dysagrion species, considering the prominent role they have played in understanding their relationships at various taxonomic levels, we examined new, high resolution photographs of Scudder’s original Dysagrion specimens housed in the collections of the MCZ. The location of the holotype of D. packardii ( Fig. 4C View FIGURE 4 , re-drawn here from Scudder 1890, plate 6 Fig. 3 View FIGURE 3 ) is unknown, but we were able to examine new photographs of cotype MCZ 4656 (Packard number 252) ( Fig. 4A, B View FIGURE 4 ). In this specimen, the proximal portion of the antenodal region is missing, but the region where these crossveins should occur, if present, is reasonably well preserved. We did not detect any (either dry or wetted with ethanol), consistent with Scudder’s original description and contra subsequent authors. We also examined new photographs of the D. lakesii Scudder (Green River Formation, Wyoming) type specimen MCZ 4101 (Packard number 259, not previously illustrated) ( Fig. 5 View FIGURE 5 ); these also show no evidence of such accessory crossveins, which should be readily detectible if present.
In the type of D. fredericii (Scudder’s number 4167/4168, now MCZ numbers 381A, B), we see one weakly preserved accessory crossvein, which we indicate by a dotted line in Fig. 6 View FIGURE 6 , but we doubt there are two, differing from Scudder’s drawing (1890) as discussed by Cockerell (1908a). Tillyard & Fraser (1939) and Fraser (1957: Fig. 34.2 View FIGURE 34 , spelled “ frederici ”) subsequently reproduced Scudder’s drawing of this specimen with two accessory crossveins. We also examined photographs of MCZ 382, 1497, and 4147, but these fossils are fragmentary and do not possess this region. Cockerell and subsequent authors (as above) were then incorrect in asserting that the presence of accessory antenodal crossveins characterises Dysagrion and justifies its association with Phenacolestes .
Seven genera have been subsequently associated with Dysagrion and Phenacolestes as forming the current Dysagrionidae : Primorilestes , Petrolestes , Congqingia , Electrophenacolestes Nel & Arillo , Burmadysagrion Zheng et al. , Electrodysagrion , and Palaeodysagrion (we exclude the latter three, see below) ( Figs. 7–9 View FIGURE 7 View FIGURE 8 View FIGURE 9 ). None of these except Electrophenacolestes possess an accessory antenodal crossvein. None of the new species that we associate here with the family (below) possess them. A presence of accessory crossveins is not, therefore, a useful character state on which to base this family concept, define constituent subfamilies, nor characterise the genus Dysagrion .
Subsequent definitions of Dysagrionidae . Some or all of the above genera and sometimes Thaumatoneura , Eodysagrion Rust et al. , and others have been grouped in various family configurations, including in the Agrionidae , Amphipterygidae , Pseudolestidae , Megapodagrionidae , as subfamilies of Thaumatoneuridae ( Dysagrioninae and Thaumatoneurinae: e.g., Bechly 1996; Nel et al. 2005 a, 2005b; Nel & Arillo 2006), or of the Dysagrionidae ( Dysagrioninae , Thaumatoneurinae and Eodysagrioninae: Rust et al. 2008; Dysagrioninae , Burmadysagrioninae and Eodysagrioninae: Zheng et al. 2016a, 2016b, 2017; and independent of Thaumatoneura and Eodysagrion: Garrouste & Nel 2015 ; Nel et al. 2016; Huang et al. 2017).
Bechly (1996) distinguished the taxon as Dysagrioninae (within Thaumatoneuridae : Dysagrion , Petrolestes , Phenacolestes and Congqingia ) by quadrangle shape as its single defining trait, although he did not describe this, and noted that it is also present in Sieblosiidae . Nel & Arillo (2006) agreed, further distinguishing it by the combination of: antesubnodal space without crossveins; absence of the oblique vein O; CuA–A space very broad; the base of IR2 positioned proximal to the nodus; RP3-4 arising between the arculus and nodus closer to nodus; and the base of RP2 being in a very distal position. Nel & Fleck (2014) closely followed this definition.
Rust et al. (2008) erected the Eodysagrioninae for Eodysagrion as an equal rank taxon with Dysagrioninae and Thaumatoneurinae within the Dysagrionidae .
The Dysagrionidae is currently not considered to be close to the Thaumatoneuridae . Garrouste & Nel (2015) questioned a close relationship between them because of inconsistencies in positions of the nodus and bases of branches of RP and IR2, the difference in shape of their quadrangles and of their leg spines and thoracic skewedness, and as an incomplete interpleural suture is present in Petrolestes , but it is complete in Thaumatoneura . They further suggested that Dysagrionidae might not even belong in the Zygoptera . Huang et al. (2017) explicitly separated them, elevating Dysagrioninae and Thaumatoneurinae to family level. They rejected their putative synapomorphies of costal margin curvature and lack of crossveins in the antesubnodal space as weakly supported and most likely convergent, also discussing their difference in quadrangle shape. They further considered Eodysagrion as a provisional member of the Thaumatoneuridae , among their reasons further stressing the importance of quadrangle shape, which is rectangular in Eodysagrion like that of Thaumatoneura , not Dysagrionidae .
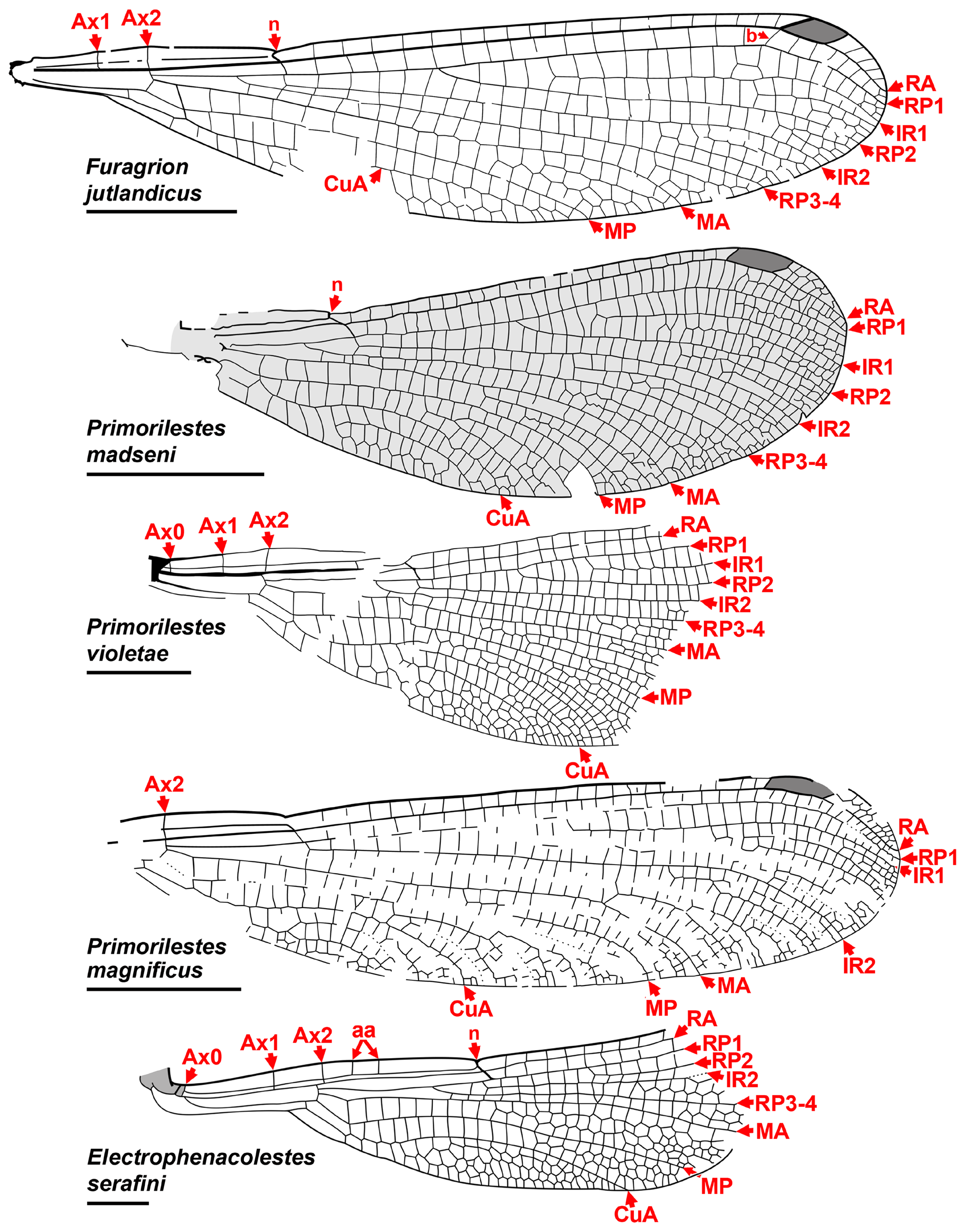
Subfamilies of Dysagrionidae . Huang et al. (2017), in elevating the Dysagrioninae to family status, also elevat- ed the former tribes Petrolestini and Dysagrionini to subfamilies: Petrolestinae, comprised of Petrolestes ( P. hendersoni Cockerell : Ypresian Green River Formation, Colorado, United States of America; P. messelensis Garrouste & Nel : Lutetian, Grube Messel, Germany) and Congqingia ( C. rhora, Aptian , i.e., Early Cretaceous, Laiyang Formation, Shandong, China; recent age estimate: Zhou et al. 2020) ( Fig. 8 View FIGURE 8 ); and their revised concept of Dysagrioninae , comprised of Dysagrion , Phenacolestes , Primorilestes ( P. violetae Nel et al. : Priabonian, Bolshaya Svetlovodnaya (Biamo), Primorye, Russia; P. madseni Rust et al. : earliest Ypresian Fur Formation, Denmark; P. magnificus Nel et al. : Miocene, Satovcha Graben, Sivik Formation, Bulgaria), and Electrophenacolestes ( E. serafini: Priabonian, Baltic amber, Poland) ( Fig. 7 View FIGURE 7 ).
Bechly (1996) separated these (as tribes) by Petrolestinae having: 1, no accessory antenodal crossveins; 2, IR1 shortened and strongly zigzagged; and 3, the bases of RP3-4 and IR2 situated midway between nodus and arculus [ Dysagrioninae : origin of RP 3-4 in middle third between arculus and nodus, usually at about two-thirds the distance; IR2 originates at or very near subnodus]. However, character state 1 is, as above, variable and not informative. Nel et al. (2005b) rejected character state 2, as this region is not preserved in the type species Petrolestes hendersoni and is not consistent in other specimens. Character state 3 is the sole trait separating these taxa. In the Dysagrioninae the origin of RP3-4 is in the middle third between the arculus and nodus, usually at about two-thirds of the distance, and IR2 originates at or very near the subnodus. In the Petrolestinae, the origin of RP3-4 is more proximally positioned, closer to or at the point midway between the arculus and subnodus, and IR2 originates close to or on RP3-4. There are no currently recognised taxa of tribe-level rank of the Dysagrionidae .
Emended diagnosis of the Dysagrionidae . The wings of Dysagrionidae are easily separated from those of other Cephalozygoptera and tentative Cephalozygoptera (new suborder, see below) by a combination of:
1- oblique crossvein O absent [ Sieblosiidae : present];
2- arculus at or immediately proximal to Ax2 [ Whetwhetaksidae : nearer to Ax1].
They are distinguished from similar appearing Zygoptera by a combination of the above and the following character states 3–9 of the wings, slightly amended from the diagnoses of Nel & Arillo (2006) and Nel & Fleck (2014), and 10, of the head:
3- quadrangle broad, distal side longer than proximal side, posterior side longer than anterior, distal-posterior angle oblique, proximal-anterior angle usually about 90°;
4- nodus positioned at least a quarter wing length, usually more;
5- anterior anal vein separates from posterior anal vein briefly before joining CuP ( i. e., is briefly free distal to petiole);
6- RP3-4 originating roughly between one third to two thirds length from arculus to subnodus, usually about two thirds ( Primorilestes violetae furthest, just distal to middle third, but P. madseni within middle third);
7- antesubnodal space without crossveins;
8- CuA–A space expanded in middle to at least two cells wide, often more;
9- CuA long, terminating on posterior margin at mid-wing or longer (termination most proximal in Primorilestes species, but there mid-wing);
10- head width across eyes about twice the length from anterior margin of antefrons to posterior of occiput; compound eyes more or less adpressed to the head capsule, convex laterally but not spherical, posterolateral corners extended posteriorly to varying degrees, sometimes even acutely; distance between compound eyes at level of centre of ocelli about one eye’s width or less; head not distinctly extended laterally with bulging, spherical compound eyes as in Zygoptera ( i.e., not “hammerhead” or “dumbbell” shaped).
We agree with Nel & Arillo (2006), Nel & Fleck (2014), and Garrouste & Nel (2019) that an expanded CuA–A space is characteristic of the Dysagrionidae (character state 8).
These character states associate Dysagrion , Phenacolestes , Primorilestes , Petrolestes , Electrophenacolestes , and Congqingia in agreement with previous authors, as well as Furagrion Petrulevičius et al. (restored, below); the new genera described here, Okanagrion , Okanopteryx and Stenodiafanus ; and species of the new collective genus Dysagrioninites. They exclude Electrodysagrion , Palaeodysagrion and Burmadysagrion (see below). We tentatively include the Thanetian (late Paleocene: see age discussed by Wedmann et al. 2018) Valerea Garrouste et al. (one species: V. multicellulata, Menat Formation , France) for reasons discussed below.
We include Furagrion in the Dysagrionidae and treat Valerea as a tentative member. Henriksen (1922) originally described Furagrion jutlandicus (Henriksen) from the early Ypresian Fur Formation of Denmark (“Mo-Clay”) as a species of Phenacolestes , and therefore a member of Cockerell’s Dysagrioninae . He based it on a specimen with three partial wings and part of the abdomen. None of these wings are preserved proximal to the nodus, and although they then lack the quadrangle and antenodal space, he associated the species with Phenacolestes by shared character states of the preserved portion. Owing to the lack of the proximal wing characters, Nel & Paicheler (1994) treated the species as “ Phenacolestes ” jutlandicus of indeterminate family. Rust (1999) reported a new, almost complete wing which he called “ Dysagrioninae gen. indet. jutlandicus ”. Petrulevičius et al. (2008) revised the species based on this fossil, erecting the monotypic genus Furagrion for it. They assigned it to the Megapodagrionidae , finding an association with the Dysagrionidae unlikely by the lack of a distinct broadening of the antenodal area at the level of Ax1 and Ax2, and the distal side of the quadrangle not distinctly longer than the proximal. However, the amount of broadening of the antenodal area is variable in the family, and is in some species quite slight, and so this character state does not have clear diagnostic value in distinguishing it; in Furagrion it is comparable with that of Electrophenacolestes . While the distal side of the quadrangle of Furagrion is not much longer than the proximal side as it is in many dysagrionids, it is still longer, like that of Congqingia ( Fig. 8 View FIGURE 8 ). Furagrion then satisfies all wing character states of the diagnosis provided above, and so we restore it to the Dysagrionidae . Zessin (2011) described a second species of the genus, F. morsi Zessin , based on the proximal half of a wing.
Although the enigmatic Thanetian Valerea multicellulata ( Fig. 9 View FIGURE 9 ) is known from a single, fragmentary wing in which only character state 1 of our family definition is determinable, we tentatively associate it with the Dysagrionidae by its otherwise notable similarity with the new genus Okanagrion with which it shares distinctive character states, some not otherwise known in the Odonata (see below, in the discussion of Okanagrion and support by our cladistic analysis).
The Alaskan Chickaloon specimen and “ Megapodagrionidae ” genus and species A are probably dysagrionids; Thanetophilosina Nel et al. and NHMUK I.9866/I.9718 could be dysagrionids. We agree with Garrouste & Nel (2019) that the fragmentary specimen from the Thanetian or Ypresian Chickaloon Formation of Alaska (unnamed) is likely a dysagrionid by its density of veins and long CuA with an expanded CuA–A space up to four cells deep ( Fig. 9 View FIGURE 9 ). Its preserved portion only possesses character states 1, 8 and 9 of the Dysagrionidae diagnosis, and so we treat it here as a probable member of the family.
Similarly, the partial wing “ Megapodagrionidae ” genus and species A of Petrulevičius et al. (2008) from the early Lutetian of Grube Messel, Germany, shares character states 1, 8 and 9 of the Dysagrionidae diagnosis with the Chickaloon specimen, and is more complete than it, with the apical portion present and well-preserved. Petrulevičius et al. (2008) discuss various possible family associations and only tentatively assign it to the Megapodagrionidae , noting that as then constituted, that family could not be defined by any known synapomorphies of wing venation. The Megapodagrionidae , long considered to be polyphyletic, has since been separated into a number of families based on a revised molecular phylogeny ( Dijkstra et al. 2014). We treat this fossil as also probably a dysagrionid, cf. Dysagrionidae genus and species A ( Fig. 9 View FIGURE 9 ).
Nel et al. (1997) assigned Thanetophilosina menatensis from the Thanetian of France ( Fig. 9 View FIGURE 9 ) to the Megapodagrionidae , rejecting Dysagrionidae based on its differences with Dysagrion , however, knowledge of the family has increased in subsequent decades; it does conform to character states 2 through 5 and 7 of the diagnosis provided here. It is too incomplete to assess the other character states of the diagnosis and we treat it as a possible dysagrionid of indeterminate family affinity.
Specimen NHMUK I.9866/I.9718 ( Fig. 9 View FIGURE 9 ), “Dysagrionini” species A of Nel & Fleck (2014), a fragmentary proximal portion of a wing from the Priabonian of the Isle of Wight, could also belong to the Dysagrionidae . The authors assigned this species to the family and Dysagrioninae (then Dysagrionini) by an inferred broad CuA–A space, the position of the base of IR2 relative to the nodus, and the shape of the quadrangle. The quadrangle is of the Dysagrionidae type and the origin of IR2 conforms with its position in the Dysagrioninae as then defined, however, a possible broadening of the CuA–A space is not clear to us. This quadrangle shape is also found in the Sieblosiidae , the new family Whetwhetaksidae , and at least some Frenguelliidae (see below). Note the presence of Ax0, found in the Dysagrionidae , Sieblosiidae and Whetwhetaksidae . It is excluded from the Sieblosiidae and Whetwhetaksidae by the position of the origin of IR2. Although it is most like the Dysagrionidae , the origin of RP3-4 is closer to the subnodus than it is in any member of the family; only Primorilestes violetae approaches this condition, but even there it is not so close; if NHMUK I.9866/I.9718 is a member of the Dysagrionidae , it differs strongly by this.
Burmadysagrion, Electrodysagrion, and Palaeodysagrion are not dysagrionids. These three genera from mid- Cretaceous Burmese amber ( Fig. 9 View FIGURE 9 ) were assigned to the Dysagrionidae by their authors; however, they do not conform with its diagnosis.
In the wing of Electrodysagrion lini Zheng et al. (Cenomanian, Myanmar) ( Fig. 9 View FIGURE 9 ), the pterostigma is hyaline and rectangular with straight sides, unlike those of all known dysagrioninds. Although almost the entire CuA–A space is missing in both preserved wings, the authors inferred by its almost straight, very slightly curved CuA that it had a single row of cells with a slight broadening of the CuA–A space distally. Zheng et al. (2019) described Electrodysagrion neli , a second species of the genus, which has a single row of cells in the CuA–A spaces of its fore- and hind wings. This contradicts character state 8 of the Dysagrionidae diagnosis, that the CuA–A space is expanded in the middle, two or more cells wide.
The CuA–A space of Burmadysagrion ( B. zhangi, Cenomanian , Myanmar) ( Fig. 9 View FIGURE 9 ) is also narrow with a single row of cells. Further, its quadrangle is wider proximally than distally, far from its distinctive shape found in the Dysagrionidae (see diagnostic character state 3) ( Zheng et al. 2016a).
Zheng et al. (2016b) noted that the distinctly elongate—length over two and a half times its maximum width— and nearly rectangular quadrangle of Palaeodysagrion cretacicus Zheng et al. (Cenomanian, Myanmar) differs from that of all known Dysagrionidae , and recognized that it does not fit either of its established subfamilies. Huang et al. (2017) suggested that the phylogenetic positions of Palaeodysagrion and Burmadysagrion should be revised and commented that in their opinion, the only Mesozoic dysagrionid is Congqingia ( Electrodysagrion , also published in 2017, was not included in their consideration). The more complete Palaeodysagrion youlini Zheng et al. ( Fig. 9 View FIGURE 9 ) was subsequently described, in which the entire CuA–A space is one cell wide, similar to the that of Burmadysagrion ( Zheng et al. 2018) .
For these reasons, we treat Palaeodysagrion , Electrodysagrion and Burmadysagrion as zygopterans of undetermined family affinity. This is further supported by our cladistic analysis, see below.
Suborder placement of the Dysagrionidae
There has been some suggestion that the Dysagrionidae might not belong to the Zygoptera ( e.g., Cockerell 1927; Zhang 1992; Garrouste & Nel 2015). The following character states were considered diagnostic of the Zygoptera by Bechly (1996: character states 1, 2, 4–7, 12), Rehn (2003: 1–2, 8–12) and Fleck et al. (2004: 3):
1- head capsule anterio-posteriorly compressed and transversely elongate;
2- eyes dorsally separated by at least the width of one of them, usually by considerably more, eye width almost always less than a third of head width (we emend this, below);
3- ocelli arranged in a close equilateral triangle on the same plane;
4- fore- and hind wings petiolate;
5- fore- and hind wings with the same shape;
6- fore- and hind wings wing with the same venation;
7- crossvein Ax0 missing or obscured by sclerotization;
8- midfork (branching of RP1-2 and RP3–4) at less than a quarter wing length;
9- origin of IR2 at less than a quarter wing length;
10- arculus at or immediately proximal to Ax2;
11- males with well-developed pair of ventral paraprocts at the apex of the abdomen that, along with a pair of cerci, function to grasp the female during mating;
12- the ligula as a multisegmented copulatory organ in the secondary male genital apparatus.
Character state 12 and others of the Zygoptera provided by these authors ( e.g., spine density on some veins or those of larvae) are not preserved in these fossils. The amount of thoracic skewedness ( Rehn 2003) could not be confidently assessed on the few fossil specimens available and in any case may not be informative (Fleck et al. 2004; Garrouste & Nel 2015).
Bechly (1996: page 361) characterises the eyes as “very widely separated”, and Rehn (2003: page 217) that the distance between them is greater than their own width. We find that they are separated by about twice the eye width or more ( Table 2 View TABLE 2 ). Bechly (1996), Rehn (2003), and Fleck et al. (2004) list instances of reversals and homoplasies of all wing and many body character states proposed to characterise the suborder. Some appear plesiomorphic with regard to Odonata . Head/eye morphology and that of the ligula are then of primary importance in defining the Zygoptera concept.
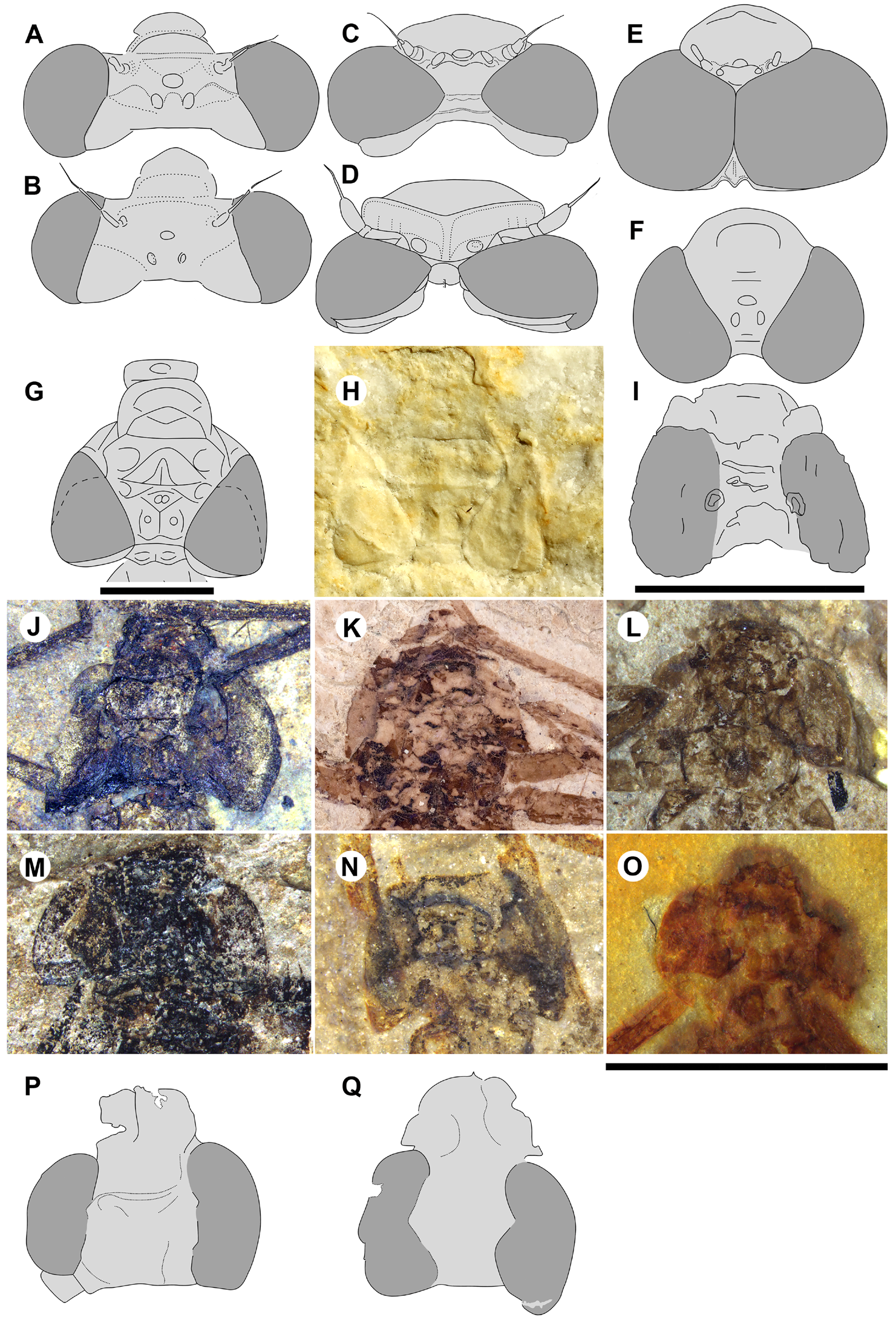
Garrouste & Nel (2015, Fig. 4 View FIGURE 4 ) noted that the head is stout, not as in Zygoptera , in the only specimen of Petrolestes hendersoni with the head preserved. They recognized that this has implications for its suborder affinity, and by extension for that of Congqingia and possibly Dysagrion . The compound eyes of P. hendersoni do not bulge out from the head as much as they do in Zygoptera , but are adpressed to it to form a more compact structure; although the inner eye margins are somewhat unclear, the distance between them is roughly 1.3 times the width of an eye ( Table 1 View TABLE 1 ). Garrouste & Nel presumed that this morphology as preserved may be due to post-mortem distortion and not reflect true head shape; however, they maintained some doubt, referring the Dysagrionidae to “?suborder Zygoptera ” based on other reasons. Cockerell (1927: page 82) said of the holotype wing of P. hendersoni (the head is not present on this specimen): “it is perhaps significant that in some aspects Petrolestes reminds one of certain Anisozygoptera ” and compared it with those of Epiophlebia .
We are aware of four other previous specimens of Dysagrionidae that have the head preserved: the holotype of Congqingia rhora , two assigned to Dysagrion fredericii , and one to Phenacolestes parallelus . Zhang (1992: page 376) described the head of Congqingia as not transversely elongate but nearly semicircular, with large eyes, “not as lateral swellings”, and separated by less than their width (see Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 of Zhang 1992). This is like the head of P. hendersoni . He suggested that by this morphology, Congqingia is excluded from the Zygoptera and might belong to the Anisozygoptera . He also described (page 380) an undoubted zygopteran that “clearly shows a large transverse head, obviously wider than long, with eyes strongly projecting from the sides of the head and almost stalked …” from the same locality, showing that the distinctive damselfly head shape may be clearly preserved as such in these beds. Zhang specifically mentioned that fossils in this deposit are not distorted. Nel et al. (1993) also considered Congqingia as a possible anisozygopteran. The D. fredericii specimens were figured by Scudder (1890, plate VI, Figs. 2 View FIGURE 2 and 10 View FIGURE 10 , Packard numbers 4183 and 4179, reproduced here as our Fig. 6 View FIGURE 6 ). Number 4183 is associated with the species by the proximal portion of an attached wing, and 4179 by its great similarity to 4183. These heads show this same rounded morphology with adpressed, closely set eyes. The Phenacolestes parallelus specimen (UCM 4545 from Florissant: University of Colorado, Boulder collection) also shares this head and eye morphology (low resolution photograph published by Cockerell 1908c, his Fig. 4 View FIGURE 4 ; see our Figs. 3E View FIGURE 3 , 10K View FIGURE 10 ).
We can now evaluate the heads of the new dysagrionids reported here. Both specimens of Okanopteryx macabeensis ( Fig. 10L, 10 O View FIGURE 10 ) with the head preserved, and one each of O. fraseri ( Fig. 10N View FIGURE 10 ), Okanagrion beardi new species ( Fig. 10M View FIGURE 10 ), and Cephalozygoptera incertae sedis ( Figs. 10J View FIGURE 10 , 59E View FIGURE 59 ), all have heads like that of P. hendersoni , P. parallelus and C. rhora . Their maximum width is about 2.0 to 2.7 times the length from anterior edge of antefrons to the posterior of occiput; the mean is 2.3. In the extant Zygoptera that we examined, this width/length ratio ranges from 2.8 to 5.5 with a mean of 3.7. The eyes are adpressed to the head capsule, i.e., not bulging outward from it as in Zygoptera , convex laterally but not spherical, and the posterolateral corners are extended posteriorly to varying degrees, sometimes even acutely. They are narrower dorsally relative to head width and the space between them is relatively narrower than in Zygoptera . The distance between the compound eyes at the level of the centre of the ocelli is about one eye’s width or less (width between eyes/width eye has a range of 0.8–1.1, mean 0.9: Table 1 View TABLE 1 , Dysagrionidae only), more closely set than in the Zygoptera ( i.e., contradicts character states 1 and 2). This cannot be explained as an artefact.
Insects preserved as compression fossils in shale invariably undergo mechanical damage, and sometimes plastic distortion during and after diagenesis. Mechanical damage is that damage that is produced post-mortem but before the insect enters the substrate. It may include partial or complete disarticulation from various factors, e.g., those specimens that experience high-energy environments and so buffeting and impacts from e.g., wave action during floating on the surface of the lake or in transportion by running water to the depositional setting. While floating on the water surface, insects may be subject to scavenging, perhaps by other arthropods or vertebrates that might consume the body and reject the wings. At least some mechanical damage invariably happens, and all specimens reported here are at least somewhat disarticulated and are usually isolated wings, often incomplete. Such mechanical damage may at times cause mouthparts to be displaced, perhaps at times projected forward, which may give a false impression of head shape in some specimens; this is clearly not the case in these fossils. Further, mechanical damage would not plastically distend the head evenly along a single plane and change the shape and positioning of the eyes, much less do this in a consistent manner in all specimens.
Secondly, in less common cases there may be plastic distortion during or after the fossilization process while the insect is embedded within the substrate. Geological shear forces within the sediment may stretch the sediment with its whole insect along a discrete angle, extending it in one direction and compressing it at the right angle to this. Such plastic distortion is not an issue with the fossils that we have examined from these Okanagan Highlands localities, and has not been noted in other formations where the fossils of this group with heads have been recovered in differing depositional settings with varying diagenetic processes (Laiyang Formation, Green River Formation, Florissant Formation). In any case, such distortion would be evident in the rest of the fossil and in other insects, fish, and plants, etc. from these beds, which it is not.
By the consistency of the distinctive head and eye shape and eye positioning among all known heads of the Dysagrionidae across a variety of species and genera and the variety of formations spanning some 90 million years from the Aptian to the Priabonian, we believe this to be their true morphologies. By their distinctive differences with the conservative head and eye morphology generally agreed to be of primary importance in defining the Zygoptera , we believe this is compelling evidence that the Dysagrionidae are not members of that suborder.
Some other traits that contradict Bechly’s and Rehn’s definitions of the Zygoptera are seen in Dysagrionidae specimens where they might be detected by preservation. As mentioned above, these further traits are, however, subject to homoplasy or reversal or may be plesiomorphic; none are unambiguously diagnostic of the suborder in themselves.
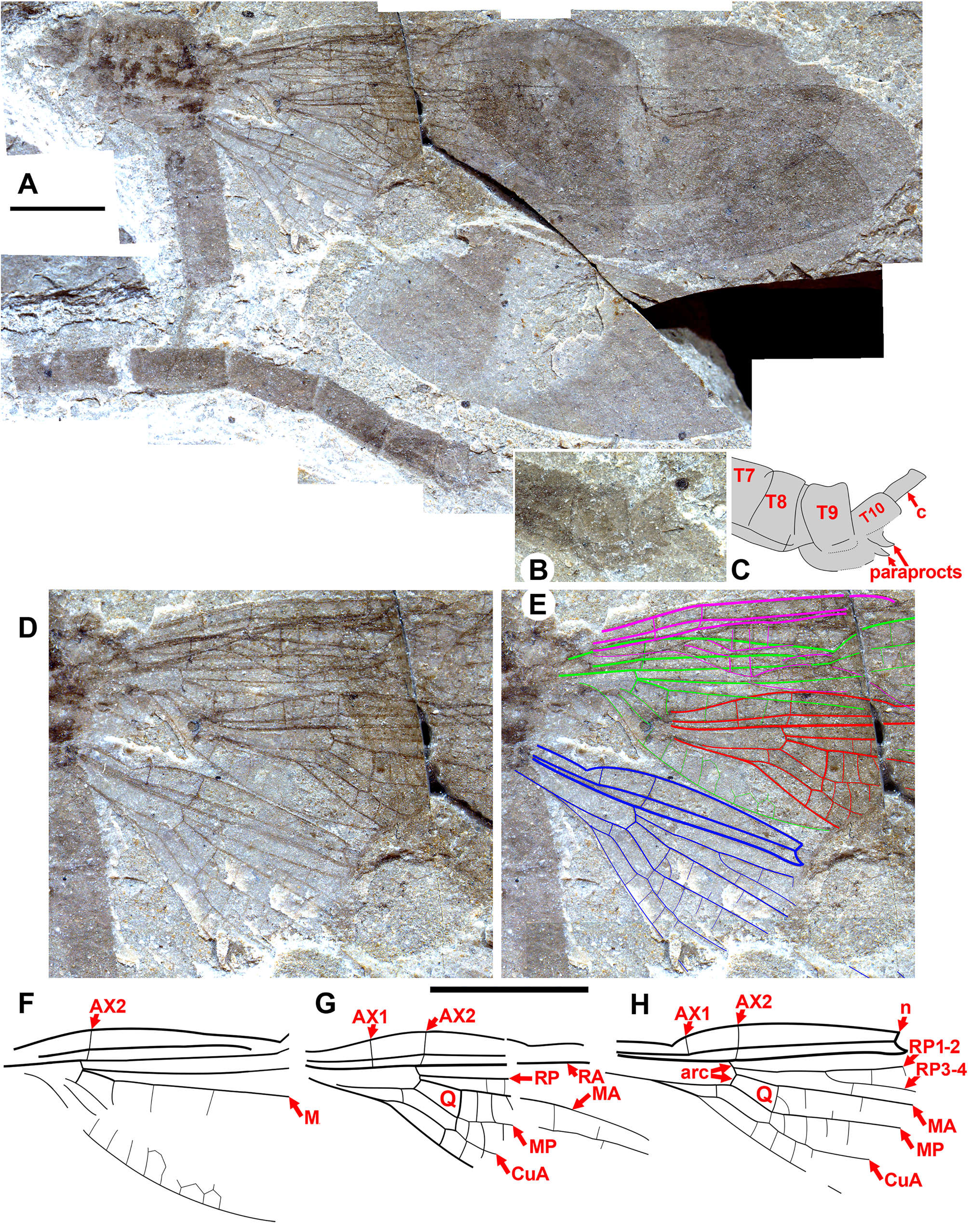
According to Bechly (1996) and Rehn (2003), crossvein Ax0 is obliterated or covered by a rather extensive sclerotization of the wing base in Zygoptera (character state 7). It is seen in some Zygoptera , however, see Litheuphaea coloradensis Petrulevičius et al. , and Labandeiraia americaborealis Petrulevičius et al. (Petrulevičius et al. 2007) (both Eocene Euphaeidae ). In the Dysagrionidae , it is seen in published drawings of the wings of Primorilestes violetae ( Nel et al. 2005b: Fig. 1 View FIGURE 1 , and see Fig. 7 View FIGURE 7 , here), Electrophenacolestes serafini ( Nel & Arillo 2006: Fig. 2 View FIGURE 2 , and see their photograph Fig. 1 View FIGURE 1 ) ( Fig. 7 View FIGURE 7 ) and Phenacolestes parallelus specimen YPMIP-220974 (see Fig. 3E View FIGURE 3 ) (and see above, specimen NHMUK I.9866/I.9718 of Nel & Fleck 2014). Ax0 is seen in the new species Dysagrion pruettae ( Fig. 11B View FIGURE 11 ), Okanagrion beardi (specimen F-790, Fig. 11A View FIGURE 11 ), Okanopteryx jeppesenorum new species ( paratype 3, SR 15-003-001, Fig. 11C View FIGURE 11 ) and possibly in some others. It may be seldom present in the Dysagrionidae as in these specimens, but it might also be more common or even ubiquitous, as the very wing base where it occurs is almost always either poorly or not preserved, or is obscured in some other fashion. For example, it is seen in the well-preserved new Okanagan Highlands species Dysagrion pruettae , but is unknown in any previous Dysagrion specimens, all of which are missing the proximal-most wing where it might be found.
Rehn (2003) found the origin of IR2 at less than a quarter wing length in many Zygoptera , with reversals and convergences. In 16 Dysagrionidae specimens that we measured, this ranged from about 30% to 38% wing length, averaging about 33%.
Garrouste & Nel (2015) found that the legs of the specimen of Petrolestes hendersoni examined bear spines shorter and stronger than in the Zygoptera , more like those present in many odonates outside of the suborder. In the Okanagan Highlands specimens, they are also relatively short, but generally weak.
The following morphology of the Dysagrionidae conforms with that of Zygoptera . The ocelli are arranged in a close equilateral triangle in the same plane, the “zygopterid type ” configuration of Fleck et al. (2004). This orientation is apparently ubiquitous in Zygoptera . Ocelli in Anisoptera form triangles which are usually far from equilateral, often strongly flattened (with the lateral ocelli farther apart and the median one moved rearward, closer to the lateral ones). However, a few, e.g., Hypopetalia McLachlan and Phyllopetalia Selys (Austropetaliidae) may have the ocelli arranged almost equilaterally (Dennis Paulson, pers. comm.). In Anisozygoptera , although the ocelli of Epiophlebia are more or less arranged equilaterally, they are much more widely separated than in Zygoptera , and the median and lateral ocelli are on different planes, with a high ridge between them. The head of Stenophlebia latreillei (Germar) (see Fleck et al. 2003; called S. aequalis by Tillyard & Fraser 1940) (Stenophlebioptera: see Fleck et al. 2004, and most recently considered in the fossil Infrasuperorder Stenophlebioptera Bechly by Huang et al. 2019) bears ocelli in this “zygopterid” arrangement ( Fig. 10F View FIGURE 10 ). This condition appears plesiomorphic with regard to the Odonata , present in the Late Jurassic and Early Cretaceous Tarsophlebiidae (Fleck et al. 2003) , sister to Odonata according to Bechly (1996) and Fleck et al. (2004) (what looks like a joined pair of median ocelli in Fig. 10G View FIGURE 10 appears to be a preservational artefact).
Male anal appendages are known in Okanopteryx macabeensis paratype 1 (GSC 141101, Fig. 54 View FIGURE 54 ) and Okanagrion hobani new species paratype 7 (F-1044, Fig. 31 View FIGURE 31 ), which bear distinctly preserved well-developed paraprocts, consistent with Zygoptera . Such paraprocts are, however, plesiomorphic to Odonata , a groundplan structure in insects. The Tarsophlebiidae possess uniquely extended appendages at the terminus of the male abdomen: either they lost paraprocts; these appendages are modified paraprocts; or they are present but not visible by chance preservation (Fleck et al. 2004).
The fore- and hind wings of Dysagrionidae are petiolate, with very close shape and venation, including quadrangles closed and not crossed, CuA is simple, the arculus is positioned at or immediately proximal to Ax2, and with a well-developed nodus; their thorax is oblique; and their bodies gracile, although these or similar conditions are present in other groups. Rehn (2003) considered the midfork at less than a quarter of wing length a derived condition, present in the Calopterigoidea and some other Zygoptera . In the remaining Zygoptera and all Anisoptera and Anisozygoptera it is in the plesiomorphic, more distal, position. In the 16 complete to reasonably complete Dysagrionidae wings as above (assuming missing bases and tips), all midforks were at about 21% to 30% of wing length, averaging about 25%. Interestingly, the eight species of the new Okanagan Highlands genera Okanagrion and Okanopteryx all had values of 26% and below, averaging 23%. According to Bechly (1996) and various subsequent authors, Dysagrionidae is in the Amphipterygoidea, i.e., in the Calopterygoidea sensu Rehn, with which this condition then mostly conforms.
TABLE 2. Head measurements of extant Zygoptera and measurement ratios: S, sex; RBCM #, Royal British Columbia Museum specimen number; WH, width of head; WBE, width between eyes; WE, width of one eye; LE, length of one eye; LH, length of head. See text for details.
| S | RBCM # | WH mm | WBE mm | WE mm | LE mm | LH mm | WH/ LH | WH/ WBE | LH/ WBE | WBE/WE | LE/WE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphipterigidae | ||||||||||||
| Amphipteryx agrioides Selys | m | 991-052065 | 6.4 | 3.0 | 1.7 | 2.7 | 1.5 | 4.3 | 2.2 | 0.5 | 1.8 | 1.7 |
| Argiolestidae | ||||||||||||
| Argiolestes griseus Tillyard | f | 991-052103 | 4.6 | 2.9 | 1.0 | 1.6 | 1.3 | 3.6 | 1.6 | 0.4 | 3.0 | 1.6 |
| Calopterygidae | ||||||||||||
| Calopteryx aequabilis Say | f | 999-002177 | 5.4 | 2.7 | 1.1 | 1.9 | 1.9 | 2.9 | 2.0 | 0.7 | 2.5 | 1.7 |
| Vestalis amaryllis Lieftinck | f | 991-052062 | 5.3 | 2.4 | 1.3 | 1.9 | 1.3 | 4.2 | 2.2 | 0.5 | 1.9 | 1.6 |
| Chlorocyphidae | ||||||||||||
| Cyrano unicolor (Hagen in Selys) | m | 988-017083 | 5.5 | 2.6 | 1.9 | 2.6 | 1.9 | 2.8 | 2.2 | 0.8 | 1.4 | 1.4 |
| Rhinocypha tincta Rambur | m | 991-052014 | 3.9 | 1.7 | 0.9 | 1.8 | 1.4 | 2.8 | 2.2 | 0.8 | 2.0 | 1.1 |
| Coenagrionidae | ||||||||||||
| Acanthagrion lancea Selys | m | 991-052186 | 3.3 | 2.1 | 0.8 | 1.3 | 1.0 | 3.3 | 1.6 | 0.5 | 3.9 | 1.7 |
| Agriocnemis femina (Brauer) | m | 988-017270 | 2.7 | 1.6 | 0.5 | 1.1 | 0.7 | 3.8 | 1.7 | 0.5 | 3.2 | 2.3 |
| Argia emma Kennedy | f | 999-002173 | 4.6 | 2.6 | 1.0 | 1.7 | 1.5 | 3.1 | 1.8 | 0.6 | 2.6 | 1.7 |
| Coenagrion pulchellum (Vander Linden) | m | 991-050943 | 3.9 | 2.6 | 0.7 | 1.5 | 1.0 | 3.8 | 1.5 | 0.4 | 3.8 | 2.2 |
| Enallagma boreale Selys | m | 998-017433 | 3.9 | 2.7 | 0.7 | 1.4 | 1.2 | 3.3 | 1.5 | 0.5 | 4.0 | 2.0 |
| Erythromma najas (Hansemann) | m | 991-050013 | 4.5 | 2.6 | 0.9 | 1.7 | 1.3 | 3.5 | 1.7 | 0.5 | 2.9 | 1.9 |
| Ischnura senegalensis (Rambur) | f | 988-011601 | 3.7 | 2.5 | 0.6 | 1.5 | 1.2 | 3.0 | 1.5 | 0.5 | 4.0 | 2.4 |
| Mecistogaster ornata Rambur | f | 991-052124 | 5.6 | 3.2 | 1.1 | 2.1 | 1.5 | 3.7 | 1.8 | 0.5 | 2.9 | 1.9 |
| Pseudagrion kersteni (Gerstäcker) | f | 991-052380 | 3.5 | 2.2 | 0.7 | 1.4 | 1.2 | 3.0 | 1.6 | 0.5 | 3.0 | 2.0 |
| Telebasis salva (Hagen) | m | 011-001340 | 3.6 | 2.4 | 0.7 | 1.4 | 1.1 | 3.3 | 1.5 | 0.5 | 3.4 | 2.0 |
| Dicteriadidae | ||||||||||||
| Heliocharis amazona Selys | m | 991-052064 | 5.4 | 2.7 | 1.4 | 2.0 | 1.5 | 3.7 | 2.0 | 0.5 | 1.9 | 1.5 |
| Euphaeidae | ||||||||||||
| Euphaea impar Selys | m | 988-017096 | 5.0 | 2.5 | 1.0 | 1.8 | 1.1 | 4.6 | 2.0 | 0.4 | 2.4 | 1.8 |
| Euphaea ochracea Selys | f | 988-011791 | 5.5 | 2.8 | 1.2 | 1.9 | 1.3 | 4.2 | 2.0 | 0.5 | 2.3 | 1.6 |
......continued on the next page
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |