Endoclita kosemponis ( Strand, 1916 ), 2019
|
publication ID |
https://doi.org/10.11646/zootaxa.4551.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:886BB4EA-3A68-47F3-BBBC-1B8D9603D13C |
|
DOI |
https://doi.org/10.5281/zenodo.5943873 |
|
persistent identifier |
https://treatment.plazi.org/id/03B5224F-C131-FFE1-FF41-F8AAFB6FFBB5 |
|
treatment provided by |
Plazi |
|
scientific name |
Endoclita kosemponis ( Strand, 1916 ) |
| status |
stat. nov. |
Endoclita kosemponis ( Strand, 1916) View in CoL , stat. rev.
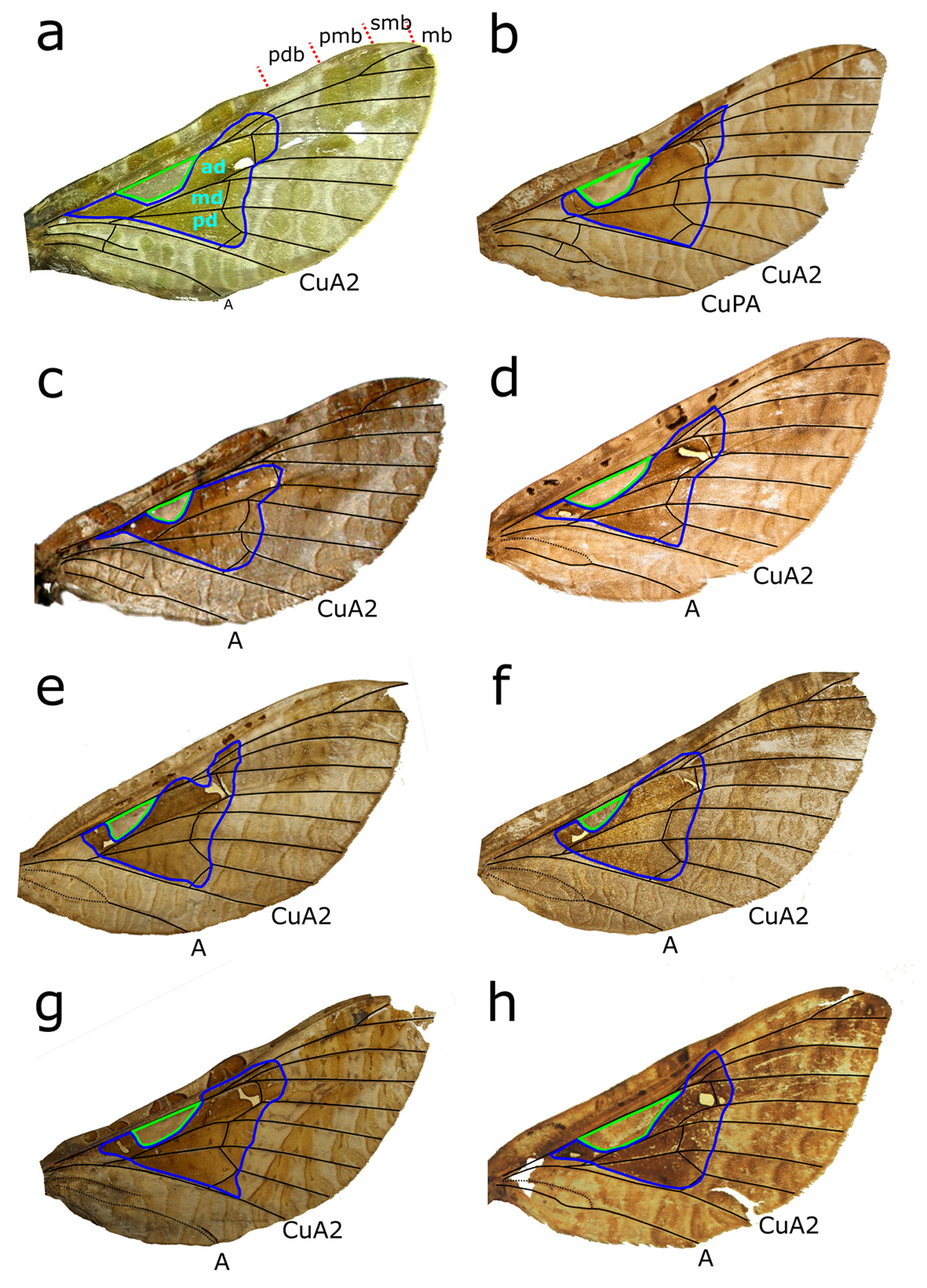
Figs. 2 View FIGURES 1–2 a–c, 4b, 5b, 6b–c, 7b, 8b,d, 9b, 10a, 11a, 12d
Phassus signifer View in CoL var (?) kosemponis Strand (1916) View in CoL ; Sonan (1938)
Endoclita kosemponis: Tindale (1958) View in CoL
Endoclita sinensis: Nielsen, Robinson & Wagner (2000) View in CoL Diagnosis. A medium sized Endoclita View in CoL species with a darker shaded triangular region over much of the discal region and a trapezoid ‘recess’ in the central anterior discal cell. These features are also present in E. chalybeatus View in CoL (northeastern India), E. davidi View in CoL ( Taiwan and western China), E. magnus View in CoL (southwestern India), E. malabaricus View in CoL (southwestern India), E. meifenga sp. n. (Taiwan), E. signifer View in CoL (northeastern India), and E. sinensis View in CoL ( Taiwan and eastern China), but not the Taiwanese species E. atayala and E. inouei View in CoL . The FW of E. kosemponis View in CoL can be distinguished from the Taiwanese triangular shaded species E. davidi View in CoL and E. meifenga sp. n. by the lack of a prominent costal lobe. The FW of E. inouei View in CoL also lacks a FW costal lobe, but can be distinguished from E. kosemponis View in CoL by the absence of the triangular discal shading and in the male genitalia by the presence of a tube-like fusion of the posterior-ventral pseudotegumen (cf. Ueda 1987). The wing pattern of E. kosemponis View in CoL is very similar to that E. sinensis View in CoL which also lacks a costal lobe. At present there is no definitive difference in wing pattern and shape to separate the two species, but they can be distinguished from each other by differences in the male and female genitalia. The pseudotegumen in the male genitalia of E. kosemponis View in CoL has a long and narrower medial posterior projection ( Fig. 9b View FIGURES 9–11 ) than E. sinensis View in CoL ( Fig. 9c View FIGURES 9–11 ) and the posterior concavity of sternum VIII in the male is deeper and more rectangular ( Fig. 8b View FIGURES 6–8 ) than in E. sinensis View in CoL ( Fig. 8c View FIGURES 6–8 ). The female internal genitalia of E. kosemponis View in CoL ( Fig. 11a View FIGURES 9–11 ) and E. sinensis View in CoL (11b) also differ with respect to the shape and relative size of the lateral caecum.
Description. Male ( Fig. 2 View FIGURES 1–2 a–b): Wingspan 71 mm; FW length: 31 mm, width: 12 mm, ratio 2.6: 1; HW length: 25 mm, width: 12, ratio 2.1: 1. Antenna filiform, pale yellowish brown, scape cylindrical, pedicel rounded and slightly barrel shaped. Flagellum with 30 annuli. Interocular-antennal scales absent.
Head. Eyes prominent. Antenna filiform, pale yellowish brown, flagellum with 30 segments. Interocularantennal scales absent.
Thorax. Covered with greyish brown scales, scutum III dark brown, anteriorly free of scales; FW triangular, costal margin without costal lobe, almost straight from humeral vein to outer third of wing where distance between margin and Sc narrows slightly; outer apical margin rounded, apex between Rs1 and Rs2; outer margin almost straight, curving posteriorly to merge with the inner margin; venation ‘hepialine’ (sensu Dumbleton 1966), Sc1 present ( Fig. 4b View FIGURES 4–5 ). FW dorsal ground colour pale reddish brown covered with darker reddish brown markings, including a large dark brown patch forming a triangular shape bounded anteriorly by R vein and posteriorly by CuA 2 and extending distally just beyond the Cu-M-Rs cross veins, and with a pale reddish brown anterior trapezoid ‘recess’ along R ( Fig. 12d View FIGURE 12 ). A premarginal band of reddish brown comprising two merged sub-bands extends transversally from CuA to the costal margin near the apex, each band comprising an elliptical shape between the veins, and darkest between costal margin and M 1. A darker brown patch also extends obliquely from the wing apex to Rs4 and M 1. A basal ovoid white spot, edged distally with dark brown is located anterior to the common stalk of M 1 +M+M 3; an elongate white stigma edged with dark brown is located on the outer anterior discal cell, basal to the Rs-M 1 cross vein with a smaller white spot located distally to the cross vein adjacent to M 1. Costal region with 5–6 dark blackish brown ovoid spots edged with pale reddish brown. Ventral FW pale greyish brown with dorsal ornamentation between costal margin and Sc; costal pocket present; and Sc lined with posteriorly oriented row of piliform scales; softer piliform scales present across much of the basal and central wing surface. Dorsal HW reddish to greyish brown Ventral HW pale greyish brown. Legs greyish brown; leg length ratio pro: meso: meta 1: 1.2: 0.66; proleg lacking epiphysis; metaleg only ¾ length of proleg, metatibia with pale yellowish brown androconia and large androconial gland along length of tibia ( Fig. 5b View FIGURES 4–5 ).
Abdomen. Greyish brown, tergites and sternites moderately sclerotized; tergum II with lateral ridge of extending anterio-medially to lateral tuberculate plate, anterior ridge extending from tergosternal connection to dorsal median, not fused across median; tergosternal connection ( Fig. 7b View FIGURES 6–8 ) with short lateral and dorsal arms and broadly triangular central region. Segment II pleurum with lateral pouch, sternum II elongate and subrectangular, with broadly v-shaped ‘waist’ posteriorly and anterio-lateral arms with sclerotized ridge, ridge extending posteriorly to waist; apical region of lateral arm strongly concave; tergum VIII trapezoid; sternum VIII longer than wide, posterior margin concave and forming two elongate posterior lateral arms ( Fig. 8b View FIGURES 6–8 ).
Male g enitalia ( Fig. 9b View FIGURES 9–11 ). Tegumen (intermediate plate) elongate. Saccus (vinculum) narrowly triangular, almost straight laterally, posterior margin with central triangular tooth. Tergal lobes not observed. Pseudotegumen well developed, margin adjacent to anogenital field smooth and strongly sclerotized, ventrally fused across median as a strongly sclerotized ventral W-shaped bridge forming the ‘ventral apical nexus’ across the median; margin of ano-genital field with medial posterior projection as a narrow spine subtended by a posterio-ventral flange, and a triangular dorsal posterior projection. Fultura superior membranous, fultura and inferior sub-square with concave lateral edges. Valva small relative to pseudotegumen, narrow, and digitiform with rounded apex. Phallus membranous, without cornutus.
Female ( Fig. 2c View FIGURES 1–2 ): Wingspan 87 mm; FW length: 43 mm, width: 17 mm, ratio 2.5: 1; HW length: 34 mm, width: 15 ratio 2.3: 1.
Head. As for the male except for smaller eyes. Antennae broken.
Thorax. As for the male except for three dark brown elongate costal spots on outer costal region and absence of metatibial androconia. Legs not dissected.
Abdomen. Pleura II without lateral pouch, sternum II damaged, lacking anterior section ( Fig. 6c View FIGURES 6–8 ); tergum VIII rectangular, wider than long; sternum VIII wider than long, posterior margin straight, anterior margin of sclerotized plate irregular, central region not reaching anterior margin ( Fig. 8d View FIGURES 6–8 ).
Female genitalia ( Figs 10a, 11a View FIGURES 9–11 ). Dorsal plate (tergum IX) with upper margin shaped as a broad inverted U and anal papillae forming shallow triangular lobes without setae; subanal plates dorso-ventrally long, curving dorso- and ventro-laterally. Lamella antevaginalis tri-lobed, the central lobe with a narrow, dorsal spatulate projection. Internal genitalia ( Fig. 11a View FIGURES 9–11 ) with ductus bursae forming a narrow tube, about twice as long as the ovoid corpus bursae. Corpus bursae with a narrow tubular caecum extending laterally from centre of the corpus and extending about 1.5 times the length of corpus bursae.
Distribution. Lowland forest. Known only from the type locality. Kosempo is the former name of Juasianpu, a small hill at 380 m altitude near the town of Jiasan, Kaohsiung County, N 23.07088, E 120.59386 ( Shi et al. 2013).
Etymology. Named for the type locality, Kosempo, Taiwan ( Strand 1916).
Examined material. Only the type series comprising a single male and female. The male syntype is here designated as the lectotype with the following labels (separated by forward slashes): Kosempo, Formosa, H. Sauter 1911 / 7.VIII / Phassus signifer Wlk. ♂ v (?) kosemponis, Strand det. / Lectotypus Phassus signifer Wlk. ♂ v (?) kosemponis Strand, 1916 , Buchsbaum & Grehan 2018, des. (ZMHB) ( Fig 2 View FIGURES 1–2 a–b). The syntype female is here designated as the paralectotype with the following labels (separated by forward slashes): Kosempo, Formosa, Sauter VII 09 / Paralectotypus Phassus signifer Wlk. ♀ v (?) kosemponis, Strand 1916 , Buchsbaum & Grehan 2018, des. (ZMHB).
Remarks. The original material described by Strand (1916) comprised a single male and single female specimen. Although Tindale (1958) referred to a male type and a female allotype, he did not expressly designate the male as the primary type. Therefore, the male syntype of Endoclita kosemponis ( Strand, 1916) is designated as the lectotype and the female syntype is designated as the paralectotype in this publication.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Endoclita kosemponis ( Strand, 1916 )
| Buchsbaum, Ulf & Grehan, John R. 2019 |
E. meifenga
| Buchsbaum & Grehan 2019 |
E. meifenga
| Buchsbaum & Grehan 2019 |
E. atayala
| Buchsbaum & Hsu 2018 |
Endoclita sinensis
| : Nielsen, Robinson & Wagner 2000 |
E. sinensis
| : Nielsen, Robinson & Wagner 2000 |
E. sinensis
| : Nielsen, Robinson & Wagner 2000 |
E. sinensis
| : Nielsen, Robinson & Wagner 2000 |
E. sinensis
| : Nielsen, Robinson & Wagner 2000 |
E. sinensis
| : Nielsen, Robinson & Wagner 2000 |
E. inouei
| Ueda 1987 |
E. inouei
| Ueda 1987 |
Endoclita kosemponis
| : Tindale 1958 |
E. kosemponis
| : Tindale 1958 |
E. kosemponis
| : Tindale 1958 |
E. kosemponis
| : Tindale 1958 |
E. kosemponis
| : Tindale 1958 |
E. kosemponis
| : Tindale 1958 |
kosemponis Strand (1916)
| , Strand 1916 |