Chrysodema (Chrysodema) eximia berliozi Descarpentries, 1948
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5214.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:1205C12E-255F-4D8D-AA3A-278750C634D1 |
|
DOI |
https://doi.org/10.5281/zenodo.7405736 |
|
persistent identifier |
https://treatment.plazi.org/id/03B7414B-1220-FFBA-07E7-FD2670C0AED2 |
|
treatment provided by |
Plazi |
|
scientific name |
Chrysodema (Chrysodema) eximia berliozi Descarpentries, 1948 |
| status |
|
Chrysodema (Chrysodema) eximia berliozi Descarpentries, 1948 View in CoL
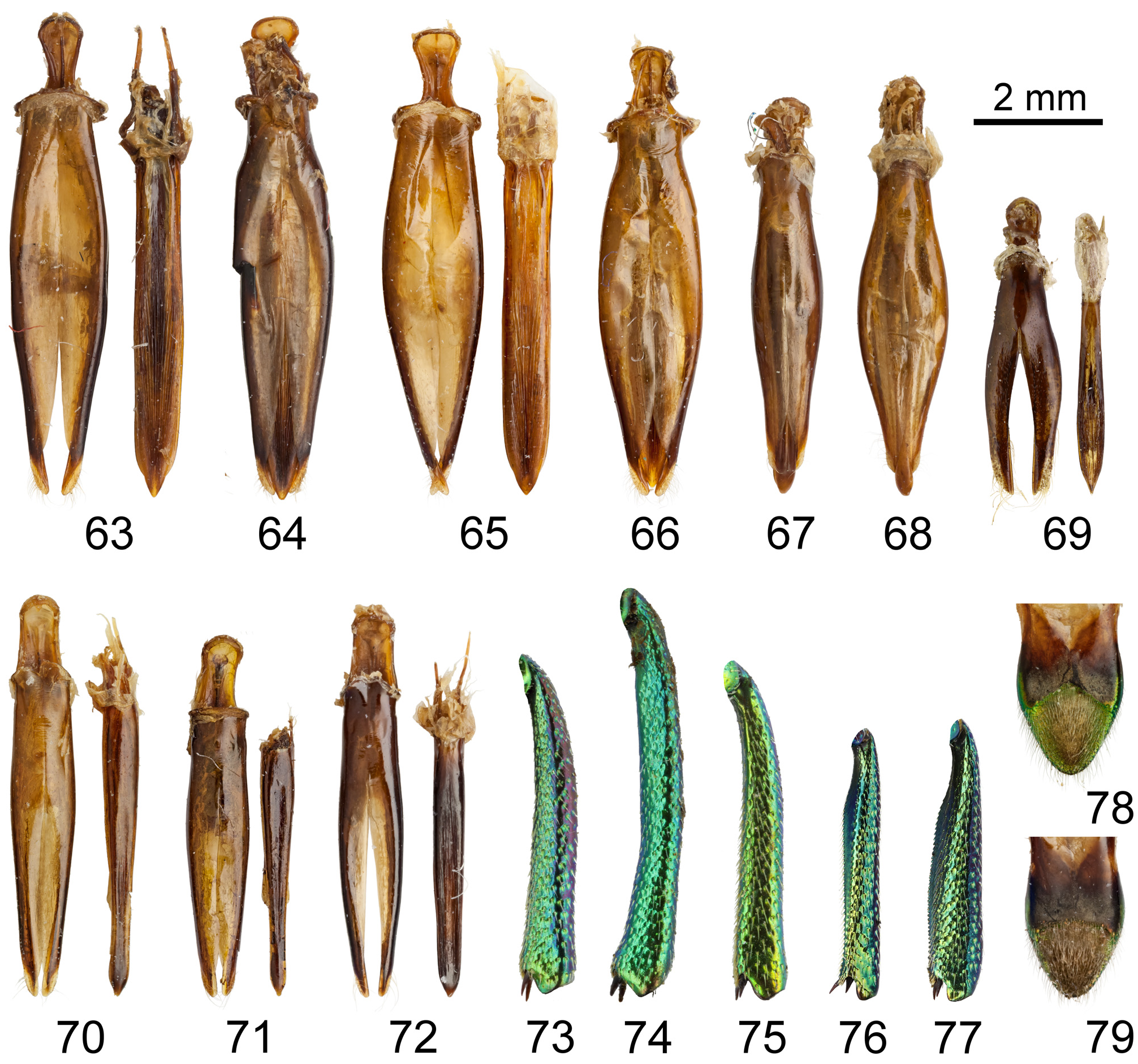
( Figs 12 View FIGURES 8–12 , 18–24 View FIGURES 13–18 View FIGURES 19–25 , 65, 66 View FIGURES 63–79 )
Chrysodema Berliozi Descarpentries (1948): 63 (original description); Akiyama & Ohmomo (2000): Pl. 46, Figs 487-1–487-4 (iconography).
Chrysodema (Chrysodema) berliozi: Kurosawa (1954) : 31 (redescription).
Chrysodema (Chrysodema) eximia berliozi: Lander (2003) View in CoL : 31 (revision, downgraded to subsp. of C. eximia View in CoL ), 78 (colour Fig. View FIGURES 48–54
52); Mühle (2003): 45 (noted); Kubáň (2006): 345 (catalogue); Bellamy (2008): 530 (catalogue); Qi, Ai & Song (2022): 361 (faunistics); Peng et al. (2022): 97 (redescription).
Chrysodema (Cyalithoides) eximia berliozi: Kubáň (2016) : 460 (catalogue); Tamadera & Yoshitake (2017): 127 (faunistics).
Chrysodema eximia berliozi: Ong & Hattori (2019) : 20 (diagnosis, iconography).
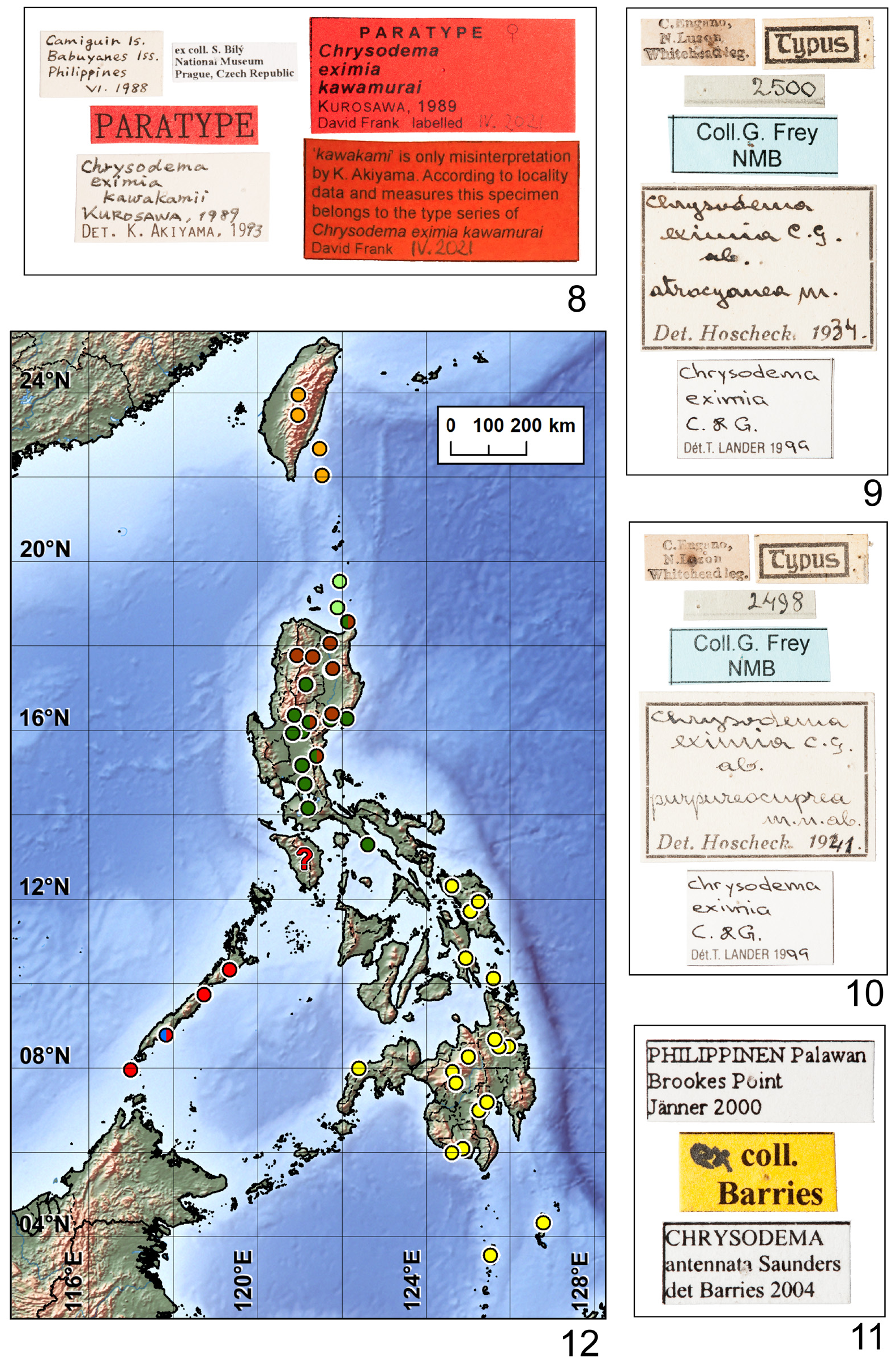
Type locality. ‘Kôtô-Sho (Botel Tobago) Formose’ [ Taiwan, Orchid Island].
Type material examined. HOLOTYPE: ♀ (34.00 × 12.00 mm), ‘KÔTÔ-ŜHO | (Botel-Tobago Is.) | FORMOSA | IV. 1936 | COLL. Y. CHUJO [w(c), h; black border] || TYPE [r, p; black border] || 16 [w(c), h] || Chrysodema | Berliozi ♀ |TYPE [red stamp] mihi | A. Descarpentries det [w, h/p]’ ( MNHN).
PARATYPE: ♀ (30.25 × 11.00 mm; Figs 19–22 View FIGURES 19–25 ), ‘ KÔTÔ-ŜHO | (Botel-Tobago Is.) | FORMOSA | IV. 1936 | COLL. Y. CHUJO [w(c), h; black border; label identical to holotype] || Chrysodema | Berliozi ♀ | PARATYPE [red stamp] mihi | A. Descarpentries. dét. [w, h/p]’ ( NMPC) .
Both specimens were provided with an additional white printed label ‘ Chrysodema (Chrysodema) | eximia | berliozi | DESCARPENTRIES, 1948 | David Frank det. IV. 2021 [date handwritten]’.
AddiTiOnal maTerial examined (4 ♂♂, 30 ♀♀). TAIWAN: Formosa, T. Kano, 1 ♀ ( MNCN, ex coll. Cobos); Yu Shan, vii.[20]06, J-M. Bousquet leg., 1 ♀ ( DFPC) ; Taiwan, Puli, Winkler, 1 ♂, 8 ♀♀ ( Fig. 18 View FIGURES 13–18 ) ( ATMR, ex coll. Novak). Orchid Island : ‘KOTOSHO | (BOTEL-TOBAGO IS.) | FORMOSA | 20. VI-10. VII 1938 | COLL. M. CHUJO [w(c), p; black border] || Chrysodema | Berliozi | mihi | PARATYPE [red stamp] | A. Descarpentries. dét. [w, h]’ 1 ♀ ( MNHN) (labeled as paratype, see ‘ Remarks’) ; Lanyu Is. , iv.1967, Coll. M. Chujo, 2 ♀♀ ( MNHN) ; Tobago, 1 ♀ ( MNHN) ; Botel-Tobago I., 2.ix.1939, T. Kaneko, 1 ♀ ( NMPC) ; Botel-Tobago I., vi.1965, 1 ♂ ( NMPC, ex coll. Bílý); Botel-Tobago I., vii.1965, 2 ♀♀ ( NMPC, ex coll. Bílý); Botel Tobako, 1 ♂ ( DFPC; Figs 23 View FIGURES 19–25 , 65 View FIGURES 63–79 ); BotelTobago, 16.v.1957, 1 ♀ ( MNCN, ex coll. Cobos); Botel-Tobago, vii.1965, 2 ♀♀ ( MNCN, ex coll. Cobos); Lanyu Is., v.1970, W. Chen leg., 1 ♀ ( MNCN, ex coll. Cobos); Lanyu Island , 25.5.1990, Coll. J. Dalihod, (3 ♀♀ NMPC, ex coll. Kubáň; 1 ♀ SGBG); Lan Yu Is., v.[19]59, 1 ♀ ( ATMR, ex coll. Novak); Lun-shu Is., 1 ♂ ( Figs 24 View FIGURES 19–25 , 66 View FIGURES 63–79 ), 2 ♀♀ ( ATMR, ex coll. Novak). WITHOUT LOCALITY DATA: R. I. Sc. N. B., I. G. 24.554, 2 ♀♀ ( IRSN) .
Redescription of paratype. Well preserved ♀ specimen, only left fore and middle tarsi partly missing. Length 30.25 mm, width 11.00 mm, length/width ratio: 2.75.
Body navicular, dorsal side metallic blue-violet with greenish reflections at apical third and green at head; ventral side (including head) and legs brown-red.
Head green on dorsal side, brown-violet on ventral side. Eyes large, oval, area around eyes finely macropunctate and sparsely pubescent. Frons green, with coppery-red reflections at lower part, 2× as wide as diameter of eye, impressed with medial sulcus, macropunctate, sparsely pubescent. Vertex green with coppery-red and bluish reflections, more coarsely macropunctate than frons. Labrum dark brown. Antennae serrate from antennomere IV. Antennomeres I and II dark green with violet reflections, sparsely macropunctate and pubescent; radicula and antennomere from III dark brown, sparsely pubescent. Antennomere II ca. 4× shorter than I and 3× than III. Antennomeres IV–VI triangular, VII–X more trapezoidal than triangular, XI oval.
Pronotum trapezoidal, narrowing anteriad with almost equal sides, widest at base, blue-violet with greenish reflections, 1.8× as wide as long. Anterior margin arcuate, lobe not protruding, densely pubescent. Disc sparsely macropunctate with micropunctures between macropunctures, with sparse short pubescence. Medial line only slightly developed but visible, flat, almost unelevated, moderately impressed at base, only bordered by macropunctures, without medial impressions. Principal impressions elongate, deep. Lateral sides from principal impressions and lateral impressions coarsely macropunctate. Lateral margin green-violet. Basal margin bisinuate.
Scutellum small, trapezoidal, shiny, violet with greenish reflections.
Elytra slightly wider than pronotum at base; parallel at basal half, narrowing from beyond its mid-length to apex; convex in lateral view. Lateral margins arcuate below humeral calli, moderately serrate at apical third. Serrations and apex of elytra violet. Four distinct main costae along suture on each elytron. 1 st costa from base to apex, 2 nd from basal fifth and joined to 1 st at apical fifth. 3 rd costa separated, shortened, from basal fifth to 3/4 length of elytra. 4 th costa also separated, from basal fifth but reaching to apex where it is almost joined to 1 st and suture. Basal fifth in front of costae 2–4 irregularly furrowed, irregularly macropunctate. Humeral calli not furrowed, irregularly macropunctate. Intercostal intervals divided by less distinct but well visible intercostae. Main costae, intercostae and top of furrows blue-violet with a few macropunctures; lower areas of furrows and intercostae intervals green with golden reflections irregularly macropunctate and with very short pubescence. Epipleura horizontal, broad at basal quarter, green-brown at basal half, violet at apical half, sparsely macropunctate and pubescent.
Legs brown-red with greenish reflections; femora less, tibiae more macropunctate and pubescent. Tibiae with two apical ventral spurs. Tarsi metallic red-brown, tarsal claws divergent and simple.
Ventral side brown-red with greenish reflections. Hypomeron irregularly macropunctate more coarsely than prosternum. Anterior margin of prosternum densely pubescent. Prosternal process subparallel-sided, narrowed at apex, approximately 2.0× as long as wide, moderately densely and coarsely macropunctate at central part, macropunctures bigger than macropunctures on prosternum, apex and sides smooth, polished and nearly impunctate. Metasternum sparsely macropunctate at central part; densely and coarsely macropunctate on sides; with short pubescence. Abdominal ventrites I–IV centrally sparsely macropunctate, laterally densely macropunctate in entire width, with short pubescence. Abdominal ventrites V densely macropunctate, with short pubescence.
Variation. Body ♂♂ (n = 4) length: 25.25–25.75(27.50) (average 26.00) mm, width: 9.25–9.75 (average 9.44) mm, length/width ratio: (2.66)2.76–2.82 (average 2.76); ♀♀ (n = 27): length: 26.50–34.00 (average 29.89) mm, width: 9.50–12.00 (average 10.85) mm, length/width ratio: 2.61–2.90 (average 2.76). Colour of dorsal part from violet to blue-black, rarely bronze-brown or green. Intercostae can be thin or only slightly indicated but always visible. Aedeagus (n = 4) length: 6.91–7.32 mm, width: 1.64 mm, length/width ratio: 4.21–4.46. Parameres very broadly regularly navicular widest at mid-length, narrowly open at apical fifth, apice of parameres rounded. Penis moderately impressed with densely arranged striate laminae at center, apex of penis broadly triangular ( Figs 65, 66 View FIGURES 63–79 ).
Differential diagnosis. Chrysodema (C.) eximia berliozi can be distinguished from the other species of this group by bicolourous head. Ventral side of head has brownish colour as well as entire ventral side but dorsal side has colour similar (or the same) to pronotum. Intercostae are always visible and most frequent colours of dorsal part are violet to blue-black. For additional characters see Key to species.
Biology. Adults feed on leaves of Terminalia catappa , sometimes found on the leaves of Mallotus japonicus ( Ong & Hattori 2019) .
Distribution. Taiwan: main, Orchid [Lanyu], and Green [Ludao] ( Tamadera & Yoshitake 2017) islands ( Fig. 12 View FIGURES 8–12 ).
Remarks. Descarpentries (1948) mentioned in his description that only two females (holotype deposited in Descarpentries’ collection and paratype from ‘même localité’ deposited in ‘Muséum de Paris’) belong to type series. The holotype is deposited in MNHN now but the paratype with the same locality label is deposited in NMPC. There is also the second female in MNHN from Botel-Tobago Is. with original Descarpentries’ type labels but with different dates of collecting. Also the Descarpentries’ (paratype) label is a little different. Therefore this specimen is not included in the type series and was provided with additional red printed labels ‘NO PARATYPE | This specimen cannot be paratype. | Descarpentries (1948: 64) mention- | ned only one paratype with the | same collecting data as holotype. | David Frank IV. 2021 [date handwritten]
Lander (2003) downgraded Chrysodema (C.) berliozi to subspecies of C. (C.) eximia . However, majority of the specimens are very different from C. (C.) eximia but one of studied specimens was metallic green and it is similar to C. (C.) intercostata (which is in synonymy of C. (C.) eximia ). Therefore it is kept as subspecies of C. (C.) eximia for the time being. It would be desirable to use molecular methods within C. (C.) eximia complex to definitively evaluate the status of this taxon (for more information see ‘Remarks’ under C. (C.) eximia ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
|
SubGenus |
Chrysodema |
Chrysodema (Chrysodema) eximia berliozi Descarpentries, 1948
| Frank, David 2022 |
Chrysodema Berliozi Descarpentries (1948)
| Descarpentries, A. 1948: 63 |