Sycorax silacea Haliday
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3737.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:24EA5BC5-2A87-4FF8-B74C-FF94590A816C |
|
DOI |
https://doi.org/10.5281/zenodo.6164567 |
|
persistent identifier |
https://treatment.plazi.org/id/03B987A6-EA28-FFC5-FF33-FC54B9E83B42 |
|
treatment provided by |
Plazi |
|
scientific name |
Sycorax silacea Haliday |
| status |
|
Sycorax silacea Haliday View in CoL in Curtis, 1839
Literature record: Krek (1999) * Sycorax tonnoiri Jung, 1953
First records for Croatia: Plitvice Lakes National park, Spring of Crna rijeka (E 15° 36' 28" N 44° 50' 14"), 28.VI.2007,emergence trap P1, 1 ♂; VII.2007, emergence trap P1, 1 ♂. MI leg.
Comments. Sycorax tonnoiri is widespread in Central and Southern Europe. Previous Balkan records of S. tonnoiri are from Bosnia & Herzegovina (Krek 1999).
Subfamily Psychodinae
Tribe Pericomaini Berdeniella keroveci Kvifte, Ivković & Klarić sp. nov.
Type material. Holotype ♂: CROATIA: Plitvice Lakes National park, Spring of Bijela rijeka (E 15° 33' 43" N 44° 50' 05"), VII.2010, (emergence trap P4), M. Ivković leg. Paratypes: same locality as holotype, VIII.2010, emergence trap P1,1 ♂; VI.2009, emergence trap P3, 1 ♂; VI.2010, emergence trap P3, 1 ♂. Plitvice Lakes National park, Spring of Crna rijeka (E 15° 36' 28" N 44° 50' 14"), VI.2006, emergence trap P4, 1 ♂; 29.V.2011, emergence trap P4, 1 ♂. All leg. M. Ivković.
Diagnostic characters. Berdeniella keroveci can be distinguished from all other Berdeniella species on the following combination of characters: Phallic sheath with deep U-shaped median incision, gonostylus s-shaped, surstylus with one distally thickened tenaculum and one to two spines. The very similar Berdeniella vaillanti (Krek, 1967) differs by having the gonocoxite with less pronounced curvature, the aedeagus with the median moveable appendage more slender and its base evenly arched, the lobes of the phallic sheath shorter and the surstyli shorter and stouter (Krek 1967, 1999; Wagner 1978).
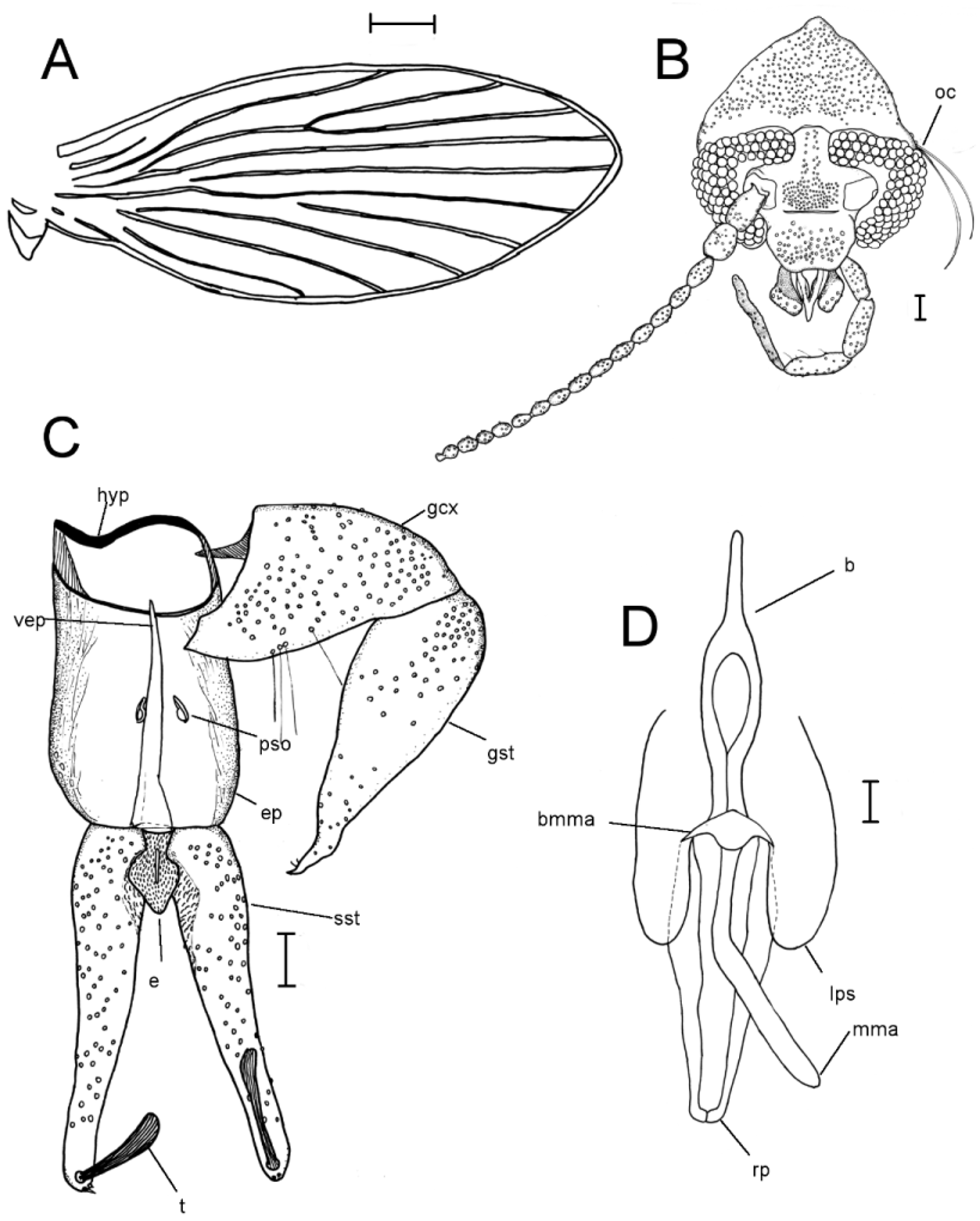
Description. Male (n = 6, except measurements which are n = 4). Head ( Fig. 1 View FIGURE 1 b) about as wide as long; vertex wide, equal to nearly half of total head length; eyebridge of four facet rows, separated by 3–4 facet diameters; ocular bristles present at sides only; interocular suture broadly U-shaped, with angular arches, occasionally reduced at middle; frontal scar patch oval with broad posterior band reaching to interocular suture; palp comprised of four segments, length of palp segments 64–80 (74): 98–108 (104): 98–118 (110): 130–172 (156); terminal palp segment striated; labellum bulbous, elongate-ovoid; antennae comprised of 16 segments; scape cylindrical, pedicel elongate-ovoid, flagellomeres fusiform; ascoids apparently absent; terminal flagellomere with stout apiculus; apiculus one third the length and half the width of the node from which it arises; length of antennal segments 72–82 (76): 62–66 (64): 54–58 (56): 40–44 (42): 38–42 (40): 36–42 (40): 38–42 (40): 36–38 (38): 36–42 (38): 36– 40 (36): 30–36 (34): 30–36 (32): 28–34 (32): 24–26 (24): 24–28 (26): 32–36 (34). Thorax with scutum, humeri, anepisternum, scutellum and lower two thirds of katepimeron densely setose, other sclerites mostly bare; legs without special features. Wing ( Fig. 1 View FIGURE 1 a) 2.26–2.4 (2.33) mm long, ovate; wing tip between R4 and R5; pterostigmal area slightly darkened, membrane otherwise clear; Sc extending to origin of R2+3; radial and medial forks about in line with apex of CuA2, but with radial fork placed slightly distal; medial fork weakened at base of M 2 in most specimens; jugum V-shaped. Terminalia with hypandrium narrow, laterally wider than at middle, often with lateral unpigmented spots or apertures; gonocoxites ( Fig. 1 View FIGURE 1 c) about twice as long as broad; shorter than gonostyli; gonostyli ( Fig. 1 View FIGURE 1 c) oblong, subconical, broadly bulbous at base, with elongate sinously curved and pointed apex, with pair of ventral setae inserted subapically; aedeagus ( Fig. 1 View FIGURE 1 d) with basiphallus narrow both in dorsal and lateral views; distal phallic appendages consisting of a moveable median morphoventral appendage and a rigid morphodorsal phallus consisting of two fused phallomeres; phallic sheath distally with two elongate lobes; base of moveable median appendage shaped like a broad arrow; epandrium ( Fig. 1 View FIGURE 1 c) about 1.5 times as long as wide, with paired pseudospiracular openings; ventral epandrial plate rod-shaped, indistinct; proctiger small, with hypoproct diamond-shaped, setose and epiproct small, ovoid; medial surfaces of surstyli basally (flanking proctiger) darker and more setose than rest of surstyli; surstyli each with one tenaculum and two spines inserted subapically; some specimens with only a single spine; tenaculum about 5 times as long as wide; apex of tenaculum expanded, smooth.
Etymology. Named after Mladen Kerovec, University of Zagreb, in gratitude for his support of MI's research in general and the Plitvice Lakes National Park emergence research in particular.
Distribution. Berdeniella keroveci has thus far only been collected from two springs in the Plitvice Lakes National Park watershed in Lika region.
Habitat. The Bijela rijeka and Crna rijeka streams are situated in the Plitvice Lakes National park in Croatia. The Plitvice Lakes system is supplied with water from the Matica stream formed by merging of the streams Crna rijeka and Bijela rijeka. The spring of Bijela rijeka is located at an altitude of 719 m a.s.l. (N 44°50'05" E 15°33'43"). It is a rheocrene spring surrounded by open canopy vegetation, and dries out only during extremely dry years (Marušić & Ćuruvija, 1990/1991). Aquatic vegetation is abundant in the spring and especially during autumn and winter allochthonous organic material is abundant due to slower stream flow. On the other hand Crna rijeka spring is a typical rheocrene spring surrounded with dense canopy vegetation. It is located at 680 m a.s.l., (N 44°50'14" E 15°36'28"). It has the highest discharge rate in the National park Plitvice Lakes, even during very dry years (Marušić & Ćuruvija, 1990/1991). The water is fast and turbulent, but very shortly the spring divides in two parts and the velocity of the water diminishes.
Variation. The examined specimens from Crna rijeka spring appear to be smaller than those from Bijela rijeka spring, but the differences are not statistically significant. The measurements reported in the description are from the type locality in Bijele rijeka spring. The two specimens from Crna rijeka spring appear to be similar, but smaller. The measurements for these specimens are, for the palps 64–66: 92–92: 96–98: 146–162; for the antennae 68–80: 60–60: 48–50: 34–36: 32–36: 32–36: 30–36: 30–34: 32–34: 30–30: 28–30: 26–28: 28–28: 22–22: 24–26: 32–32; and for the wings 1,86–2,14. It is likely that this variation is due to the differences in environmental conditions between the two springs.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Sycoracinae |
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Sycoracinae |
|
Genus |