Grammia allectans, FERGUSON
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2008.00496.x |
|
persistent identifier |
https://treatment.plazi.org/id/03B987FA-FF90-F709-FF0B-6A49FAD3E734 |
|
treatment provided by |
Felipe |
|
scientific name |
Grammia allectans |
| status |
|
GRAMMIA ALLECTANS FERGUSON ( FIGS 20, 55 View Figures 52–57 , 113 View Figures 108–113 )
Grammia allectans Ferguson, 1985: 231 , figs 55–58, 119–122.
Grammia allectans Ferguson ; Ferguson & Opler, 2006: 11.
Type material: Male holotype from 10 miles west of El Salto, 2740 m, Durango, Mexico. [ CNC, examined]; one female and 16 male paratypes [ CNC, USNM, AMNH, LACM] .
Diagnosis: Grammia allectans is somewhat similar to G. williamsii , but can be distinguished from that species by the longer antennal rami, presence of a forewing basal, medial (and often antemedial) band. In addition, the postmedial band is strongly curved basally near the costa, not straight or nearly so as in G. williamsii , and the male vesica is more kidneyshaped than globose. The ranges of these two species do not overlap, with G. allectans occurring to the south of G. williamsii in southern AZ and Mexico.
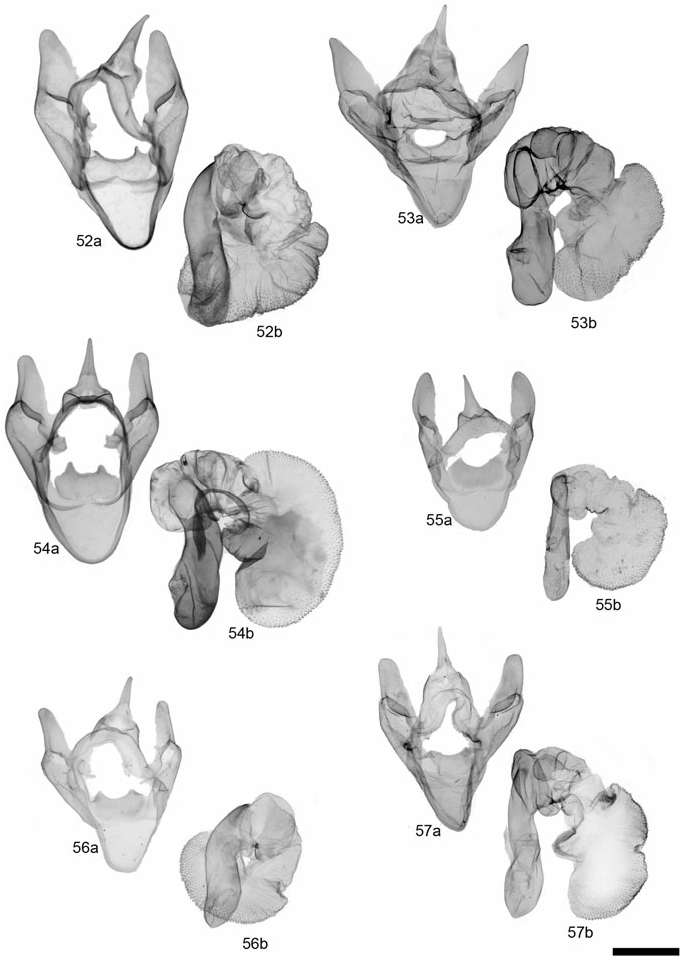
Description: Head – Frons, vertex, and palps yellowish buff, frons and palps dark brown laterally; male eyes fully developed, mean maximum diameter = 7.88 ¥ 10 - 1 mm (N = 3; AZ, MEX), but averaging larger in the AZ populations according to Ferguson (1985); female eyes 1/2 the size of male eyes [not examined; Ferguson (1985)]; male antennae strongly bipectinate, rami averaging 6.50 ¥ 10 - 1 mm, 4.13¥ longer than intersegmental distance (N = 3); dark brown and buff dorsal scales; female antennae bipectinate, branches 1.5¥ intersegmental distance [not examined; Ferguson (1985)]. Thorax – Vestiture of thorax dark brown to black, patagia, tegulae, and vertex bordered with yellowish buff; underside yellowish buff, legs predominantly pale buff. Abdomen – Ground colour pinkish-buff, pale buff near apex; medial and lateral markings dark brown to black; pale buff ventrally, dark brown to black markings consisting of two rows of spots, bordered distally at each segment margin by pale buff. Forewing – Mean forewing length 14 mm (N = 3 males); ground colour dark brown, fringe yellow-buff or dark brown; banding pattern buff to yellowish; relatively complete banding pattern; postcubital, basal, medial, postmedial, and subterminal band well developed but thinner than in G. nevadensis group, antemedial band present or absent; anal dash absent; female similar, bands slightly broader. Hindwing – Ground colour orange-red, patterned with dark brown; antemedial markings well developed, often confluent and extending basally and along anal margin; medial spot distinct; postmedial and subterminal markings usually one broad marginal band with irregular proximal border; female with dark markings less developed, not confluent; underside similar but paler in colour. Male genitalia – Distal portion of valve broad, gradually tapering to rounded apex; clasper reduced, median ridge moderately developed; uncus broadbased, process evenly tapered to point or with slight constriction near basal 1/3; juxta wider than long; aedeagus with dorsad curve at 2/3 distance beyond base; distal chamber of vesica broadly kidney-shaped, scobinate. Female genitalia – Not examined.
Biology: Adult collection dates range from early May to late June, with most records from mid to late June. The habitat where known is open montane pine forest.
Distribution: Known from the Chiricahua Mountains of AZ, and the states of Durango and Sonora, Mexico ( Fig. 113 View Figures 108–113 ).
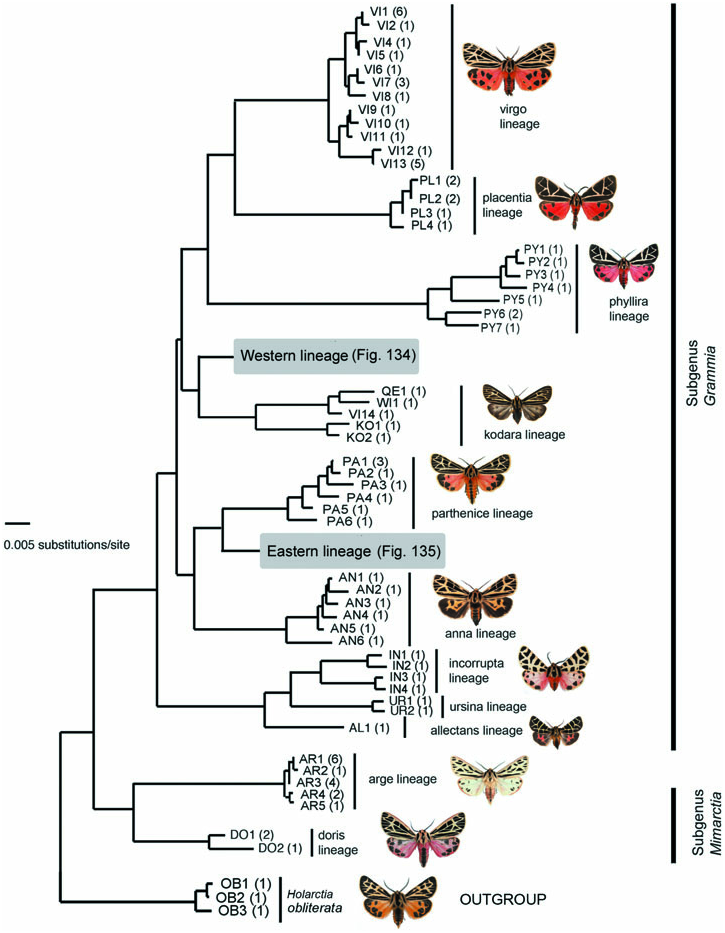
Molecular variation: The single sequenced specimen exhibited a haplotype most similar to G. incorrupta + G. ursina , forming the allectans + ( incorrupta + ursina ) lineage ( Fig. 133 View Figure 133 ).
Remarks: Ferguson (1985) states that the male valve is broader than in members of the G. nevadensis species group, but this character is quite variable even within G. nevadensis , and does not reliably differentiate G. allectans .
GRAMMIA URSINA SCHMIDT SP. NOV. ( FIGS 21, 56 View Figures 52–57 , 86 View Figures 79–86 , 114 View Figures 114–119 )
Arctia nevadensis Goater & Robinson ; Coquillett 1898: 250.
Apantesis nevadensis (Grote & Robinson) ; Medlar, 1940: 118, pl. 23.
Grammia nevadensis complex [in part]; Ferguson et al., 2000: 48.
Type material: Holotype ( Fig. 21A) – male: USA: CA, San Diego Co., MCAS Miramar , 20.ix.1998, N. Bloomfield ( CNC) . Paratypes – 54 ♂♂, 37 ♀♀. CA: San Diego Co.: NAS Miramar 6, 30.ix.1997, N. Bloomfield (1 ♂, UCB) ; NAS Miramar 3, 5.x.1997, N. Bloomfield (1 ♂, UCB) ; MCAS Miramar 2, 3.xii.1997, N. Bloomfield (1 ♂, UCB) ; MCAS Sycamore Canyon , 5.xii.1997, N. Bloomfield (1 ♂, UCB) ; Vista , 330′, 22–24.ix.1974, 4–5.x.1975, 3.x.1985 (4 ♂♂, LACM; 3 ♂♂, CNC) . Riverside Co.: Lake Matthews , 7.x.1975, J.W. Johnson (1 ♂, UCB) ; Lake Matthews , 19.x.1975 (1 ♀, UCB) ; Lake Matthews , 26.ix.–24.x.1975 [reared] (19 ♂♂ 13 ♀♀, LACM; 2 ♂♂ 3 ♀♀, CNC) ; Gavilan Hills , 19.iii.1986, F. Sala (1 ♀, UCB) ; Gavilan Hills , 4.x.1983 (1 ♂, BCSC) ; Gavilan Hills , 8–23.ix.1987, 24.ix.1987, 26.ix.1989, J. T. McBurney (2 ♂♂, LACM; 1 ♂, CNC) ; Walker Basin , 28.vii.1975 [reared] (1 ♂, LACM) ; Bundy Canyon , 1660′, 9 miles S. Perris, 18.x.1976, R.J. Ford (1 ♂, LACM) ; same data as previous, 29.ix.1975 (1 ♂, CNC) . Orange Co.: Dana Point , 26.ix.–3.x.1936 (2 ♂♂, CNC; 4 ♂♂ 5 ♀♀, LACM) ; Laguna Beach , 27.viii.–3.xi.1967 [reared] (5 ♂♂ 5 ♀♀, LACM) ; Laguna Beach , 24.ix.–22.x.1967 (1 ♂ 4 ♀♀, LACM) ; Laguna Beach , 5–9.x.1936 [reared] (2 ♂♂, LACM) ; Laguna Beach , 13.ix.1949 (1 ♂, LACM) . Los Angeles Co.: Mint Canyon , 18.ix.1950 (1 ♀ LACM) .
Etymology: The name ursina is derived from Latin meaning ‘little bear’, in reference to the dense, often dark brown thoracic vestiture of this species.
Diagnosis: The wing pattern is similar to that of Grammia nevadensis nevadensis , from which G. ursina can easily be distinguished by the remarkably long male antennal rami (> 0.75 mm vs. <0.75 mm in nevadensis ), stubbier, broader forewing shape and the pale anal dash on the forewing (very rare in G. n. nevadensis ); internally, the small corpus bursae and reduced signa 3 and 4 of the female are distinctive; the distal chamber of the male vesica are more sausage-shaped than kidney-shaped. The ranges of these two taxa are not known to overlap, with G. ursina occurring in south-western CA, including the Channel Islands.
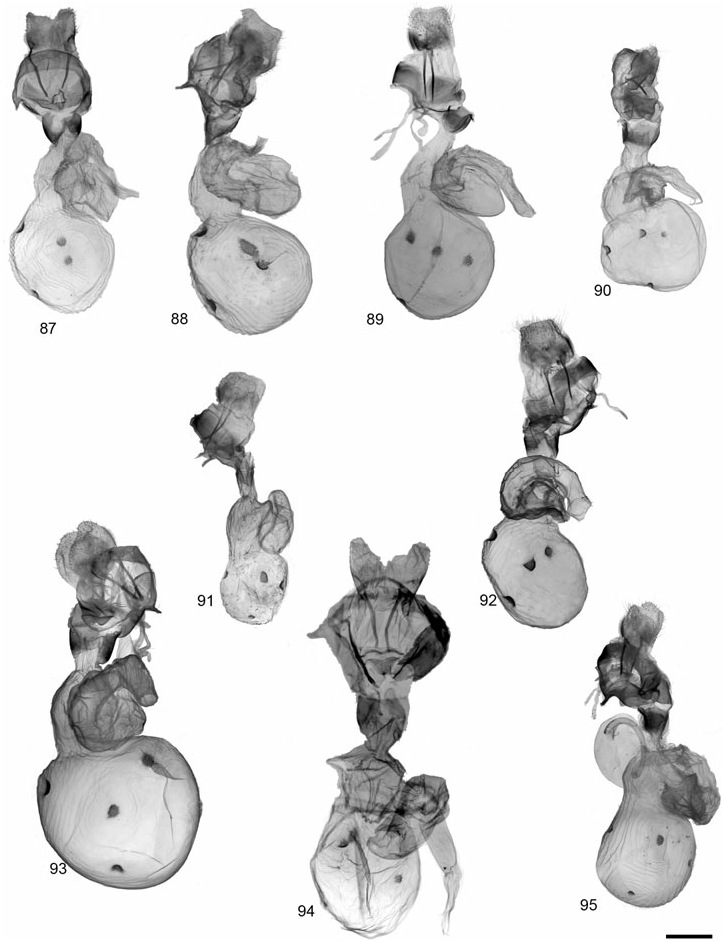
Description: Head – Frons, vertex, and palps dark brown (vertex and frons pale buff in one specimen); eyes fully developed; male antennae strongly bipectinate, rami averaging 7.91 ¥ 10 - 1 mm, 4.56¥ longer than intersegmental distance (N = 6); antenna with predominantly dark brown dorsal scales, scattered buff scales; female antennae slightly bipectinate. Thorax – Vestiture entirely dark brown to black, or with traces of slightly lighter brown borders at margins of patagia and tegula; pale-buff borders typical of the genus seen in only one of 14 specimens; entirely dark brown ventrally, or dark brown with yellowish central tuft; legs entirely dark brown or dark brown and yellowish buff. Abdomen – Ground colour pinkish-orange, pale buff near apex; medial and lateral markings dark brown to black; pale buff ventrally, dark brown to black markings consisting of two rows of spots, bordered distally at each segment margin by pale buff; females entirely black ventrally and on dorsum of last two abdominal segments. Forewing – Mean forewing length 15.3 mm for males (N = 9), females 16.7 mm (N = 2); ground colour dark brown, fringe yellow-buff to pale buff; banding pattern usually complete as in G. incorrupta and G. nevadensis , but thinner, especially in San Clemente Island specimens; bands buff to yellowish-buff; anal dash usually present; female markings similar to male, but with band colour more yellow, forewing shape slightly more elongate. Hindwing – Ground colour pale pink to orange-pink, patterned with black; antemedial and medial markings usually absent or reduced; postmedial spots well developed; subterminal markings consisting of two to three marginal wedge-shaped spots; postmedial and subterminal markings not confluent; sexes similar, with ground colour in females a richer orange-pink; ventral pattern similar to dorsum, with less intense colours. Male genitalia – Distal portion of valve relatively narrow, gradually tapering to rounded apex; clasper moderately developed ventrocaudal bulge, median ridge moderately developed; uncus broad-based, process evenly tapered to point; juxta wider than long; aedeagus with dorsad curve at 2/3 distance beyond base; distal chamber of vesica broadly kidney-shaped, scobinate, spinules relatively large as in G. incorrupta . Female genitalia – Ductus bursae unsclerotized; corpus bursae more or less globose and relatively small, 2–2.5 ¥ width of ostium bursae; signa 1 and 2 round to slightly elliptical, well developed, signa 3 and 4 reduced; appendix bursae with angled elbow, not evenly coiled; posterior apophysis 1.5 ¥ longer than length of papillae anales.
Biology: Most adult collection dates range from mid September to early October, with a few extreme records from mid August to early February. The flight dates probably represent a single generation, with some variation probably a result of annual and regional rainfall and temperature differences. Coquillett (1898) reared about a dozen larvae to adults of the black thorax form, from Santa Monica, CA; the larvae, almost certainly referable to this species, were described as follows: head black with sutures, lateral and ventral margin of clypeus, and dot at base of antennae yellow; integument black with purplish tinge, lighter grey below spiracles; dorsal line dull white, broken; tubercles light grey; setae black mixed with reddish to yellowish setae, the latter more numerous along dorsum and below spiracles; spiracles yellowish-brown ringed with black.
Distribution: Known from the Channel Islands off the coast of southern CA, although I have not seen it from San Nicholas and Santa Catalina Island; mainland south-western CA from Kern County south to San Diego County ( Fig. 114 View Figures 114–119 ), although it is likely to also occur in adjacent parts of Baja California. The record for Monterey County mapped by Ferguson et al. (2000) may also be referable to this species.
Molecular variation: Two samples of G. ursina from San Clemente Island, CA exhibited two similar haplotypes, forming the Ursina lineage ( Fig. 133 View Figure 133 ). Minimum divergence between G. ursina and G. incorrupta lineage was 3% ( Table 2).
Remarks: Although similar to Grammia nevadensis at first glance, the long antennal rami, broader forewing shape, and mtDNA haplotypes show that ursina is more closely related to G. incorrupta and G. allectans .
GRAMMIA NEVADENSIS (GROTE & ROBINSON)
( FIGS 22, 57 View Figures 52–57 , 87 View Figures 87–95 , 115 View Figures 114–119 )
Arctia nevadensis Grote & Robinson, 1866: 1 , pl. 1.
Arctia superba Stretch, 1873 [1874]: 227, pl. 9, fig. 12. Arctia geneura Strecker, 1880: 270 , pl. 9, fig. 5.
Apantesis gibsoni McDunnough, 1937: 152 .
Callarctia nevadensis form alexanderi Smith, 1938b: 10.
Apantesis nevadensis (Grote & Robinson) ; Franclemont, 1983: 117.
Apantesis geneura superba (Stretch) ; Franclemont, 1983: 117.
Grammia nevadensis (Grote & Robinson) ; Grimble, Beckwith & Hammond, 1992: 92.
Grammia nevadensis complex [in part] Ferguson et al., 2000: 48, 130.
Grammia nevadensis (Grote & Robinson) ; Ferguson & Opler, 2006: 11; Ferguson & Schmidt, 2007: 40.
Grammia nevadensis geneura (Strecker) ; Ferguson & Opler, 2006: 11; Ferguson & Schmidt, 2007: 42.
Grammia nevadensis superba (Stretch) ; Ferguson & Opler, 2006: 11; Ferguson & Schmidt, 2007: 41.
Grammia nevadensis gibsoni (McDunnough) ; Ferguson & Opler, 2006: 11; Ferguson & Schmidt, 2007: 42.
Type material: Arctia nevadensis : described from one male specimen from ‘ Nevada’ [ USA], type no. 7687 in the ANSP [not examined] .
Arctia superba : described from one male specimen from ‘ Vancouver Island’ , [BC, Canada]. The holotype male is a dissected specimen in fair condition, with missing antennae. [ AMNH, photograph examined] .
Arctia geneura : described from one male specimen from ‘Gilpin Co., Col. 8500 ft. ’ [CO, USA]. The holotype [FMNH, photograph examined] bears the labels ‘ Arctia Geneura / Gilpin Co., Col. Streck / 8500 ft. / Orig. Type. G.H.French’, ‘ A. Geneura / Streck / Gilpin Co. Col. / orig. G.H.French / Type’, ‘orig. / type.’, ‘ Arctiidae / genitalia slide / No. 1222’, ‘ HOLOTYPE [male] / det. A.Watson 1966’, ‘ Lepidoptera Type / Photograph No. 195 / Field Museum’.
Apantesis gibsoni : the holotype male is in the CNC [examined], bearing the labels: ‘ Calgary [ AB, Canada] / 7 Aug. 1902 / in light globe / T . N. Willing. ’, ‘Holo- TYPE / Apantesis / gibsoni McD / No 4209’. The specimen is in good condition, except that the abdomen is missing. McDunnough (1937) designated a female allotype from Arcola, SK (misspelled as ‘ Areola’), and a series of 23 male and three female paratypes, from Aweme, MB , Rounthwaite, MB and Calgary, AB .
Callarctia nevadensis form alexanderi: male holotype in USNM according to original description ( Smith, 1938b). An unavailable infrasubspecific name proposed for the dark thorax form.
Diagnosis: This species is widespread throughout western North America, and can be the most common Grammia species in some areas. The large amount of geographical variation can make this a difficult species to recognize, but the complete lack of forewing vein lines, complete or nearly complete complement of forewing bands, and single flight late in the season (late summer/early autumn) are good diagnostic characters. It is most similar to G. incorrupta , G. ursina , and G. bowmani , and diagnostic characters are given in each of the species accounts, in addition to those given for differences between G. nevadensis / incorrupta in Table 3. See also ‘Remarks’ below.
Description: Head – Frons, vertex, and palps with dark brown vestiture, varying to dark brown with pale buff scales on frons, apex of palps and lateral borders on vertex. Male antennae strongly bipectinate, anterior and posterior rami nearly equal in length (anterior: posterior = 0.97: 1), posterior rami averaging 6.00 ¥ 10 - 1 mm, 3.85¥ longer than intersegmental distance (N = 31; AB, SK, NV, CA, YT). Female antennae strongly biserrate to slightly bipectinate. Antennal scales dark brown, with varying amounts of pale buff scales dorsally along shaft and rami. Thorax – Vestiture of thorax, including patagia and tegula, entirely dark brown to black, or bordered with pale buff; yellow to pale buff ventrally. Leg scales entirely dark brown varying to predominantly pale buff. Abdomen – Ground colour generally orangepink, varying to pale yellow and pale buff, particularly near apex; medial and lateral markings dark brown to black, sometimes confluent but usually bordered distally by ground colour. Yellowish-buff to pale buff ventrally, dark brown to black markings consisting of two rows of spots, bordered distally at each segment margin by pale buff. Forewing – Ground colour dark brown to black, fringe white to pale buff, rarely with dark brown scales; forewing bands white to pale buff, rarely pink-buff; basal and antemedial band variably developed, present as at least a pale spot at costa; medial, postmedial, and subterminal band well developed and nearly always present; antemedial, medial, and postmedial bands, but not basal band extending caudad beyond postcubital stripe. Vein lines absent, with the exception of a basal, anal vein line expressed in some specimens, particularly prevalent in subspecies gibsoni . Hindwing – Ground colour extremely variable, ranging from white to pink, orange or yellow, although pinkish-orange in most populations; expression of dark markings variable ranging from extensive fusion of postmedial and subterminal elements to reduction of markings to only two or three postmedial spots. Male genitalia – Distal portion of valve varying from broad to slender, not constricted near middle, tapering to apex, apex rounded; clasper reduced; median ridge moderately developed; uncus broad-based, process evenly tapered to point, 2.3¥ longer than width of base; juxta slightly wider than long; aedeagus with dorsad curve at 2/3 distance beyond base; distal chamber of vesica kidney-shaped, coarsely scobinate; medial chamber 1/2 as wide as length of distal chamber, moderately scobinate; diverticula moderately developed. Female genitalia – Ductus bursae unsclerotized; corpus bursae globose to slightly pear-shaped; four round, equal-sized signa; appendix bursae with angled elbow, not evenly coiled; posterior apophysis 1.5–2¥ longer than length of papillae anales.
Biology: Adults of nevadensis generally fly in late summer, with most records from late July to mid September. Subspecies geneura flies somewhat earlier, from early July to early August, with most records from late July. The earliest dates are for the northernmost populations (subspecies vivida), with records as early as late May and June, peaking in mid to late July. There is a single annual flight. Females do not come to light and as a result are very rare in collections.
Throughout its range, G. nevadensis prefers dry, often sparsely vegetated or eroding habitats. In the northern parts of its range subspecies gibsoni is very localized in occurrence, found primarily in sandy prairie habitats such as partially stabilized dunes. Grammia n. superba is restricted to dry Garry Oak hills on Vancouver Island. Grammia nevadensis geneura occurs in mid- to high elevation dry, open forest habitat, whereas subspecies vivida occurs on dry, sparsely vegetated south-facing slopes in montane grassland, particularly on sandy soil.
Distribution: Central BC and Peace River region of AB south to eastern CA and southern NV, east to southern MB and CO ( Fig. 115 View Figures 114–119 ).
Molecular variation: Grammia nevadensis exhibits relatively large haplotype diversity with moderate divergences, although paraphyletic with respect to most species in the Western lineage. Eleven haplotypes occurred amongst the 22 specimens sampled from 16 locations across the species’ range, differing at most by 2.9% ( Table 2). Despite the paraphyletic pattern of haplotype distributions, only one haplotype was shared with another species, G. behrii , (BE3; Fig. 134 View Figure 134 ).
A short series of specimens from Hinsdale County, CO (9350″ elevation) representing subspecies geneura had haplotypes (NV9, NV10) at least 1% divergent from all other Western lineage haplotypes ( Fig. 134 View Figure 134 ), including other nevadensis haplotypes. The sampled population and topotypical geneura populations are separated by several mountain ranges and a distance of several hundred kilometres, so it is possible that the sampled population is not geneura , but another unrecognized taxon in the nevadensis group; more specimens from mid to high elevation are needed from CO.
Remarks: Grammia nevadensis exhibits a bewildering array of phenotypic variation, and mtDNA divergences unusually large for a given species. However, it seems most appropriate to treat this variation as a single species consisting of several subspecies, for the reasons outlined in Ferguson & Schmidt (2007) and the molecular variation discussed in Schmidt (2007). Briefly, the populations show clinal geographical variation into one another, and the molecular variation does not support a multispecies complex interpretation.
The superficial similarity of this species (particularly of subspecies G. nevadensis geneura ) to G. incorrupta has resulted in long-standing taxonomic confusion. Although the morphological differences are slight, the overall differences in phenotype, ecology, behaviour, and molecular variation leave little doubt that these represent different species.
GRAMMIA NEVADENSIS NEVADENSIS (GROTE & ROBINSON) ( FIG. 22A, B)
Diagnosis: This is the smallest and palest of the G. nevadensis subspecies, the hindwing white to pale pinkish-white, the subterminal band absent or reduced to one or two marginal spots, medial and antemedial spots reduced or absent (well developed in other ssp.). In most specimens, the forewing has a complete set of bands, and the thorax is entirely dark (the striped thorax form becoming more frequent in the northern and eastern Great Basin).
Distribution: The Great Basin region of the western USA, from OR and ID south through NV, east of the Sierra Nevada, CA east through UT to western CO. I have not seen specimens from AZ, but it occurs in Kane and Washington counties, UT and should occur in north-western AZ.
Remarks: Some populations from north-central OR (Madras, Umatilla, John Day River) exhibit variation from G. n. superba to G. n. nevadensis phenotypes, and populations in north-western CO (Moffat County) exhibit similar variation from G. n. nevadensis to G. n. superba and G. n. gibsoni phenotypes, including individuals intermediate between all-dark and striped thoracic vestiture, wherein the whitish stripes are darkened considerably. I interpret this variation as gradation into subspecies superba and gibsoni , respectively. The dark-thorax form is typical of subspecies nevadensis , and it apparently does not occur north of WA State.
GRAMMIA NEVADENSIS SUPERBA (STRETCH)
( FIG. 22C, D)
Diagnosis: Grammia superba is on average larger, with narrower, more yellowish forewing bands; the hindwing is bright orange-red with more extensive dark markings compared to other nevadensis subspecies. The dark-thorax form is not known to occur.
Distribution: Vancouver Island, BC, and probably also other islands in the Strait of GA.
Remarks: Although the name superba has been broadly applied to Pacific Northwest populations of G. nevadensis ( Fig. 22E, F), true G. n. superba exhibits a distinct phenotype restricted to Vancouver Island, BC, and possibly some of the adjacent gulf islands. It is disjunct from mainland G. nevadensis , the nearest populations occurring east of the coastal rain forests and Cascade Ranges in BC and WA.
GRAMMIA NEVADENSIS GENEURA (STRECKER)
( FIG. 22I)
Diagnosis: Compared to other G. nevadensis subspecies, G. n. geneura is larger with a slightly broader wing shape, and flies earlier in the summer. The hindwing ground colour is distinctly pink.
| CNC |
Canadian National Collection of Insects, Arachnids, and Nematodes |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| AMNH |
American Museum of Natural History |
| LACM |
Natural History Museum of Los Angeles County |
| CA |
Chicago Academy of Sciences |
| MCAS |
Museo Civico Archeologico e di Scienze Naturali "F. Eusebio" |
| UCB |
University of California at Berkeley |
| T |
Tavera, Department of Geology and Geophysics |
| R |
Departamento de Geologia, Universidad de Chile |
| ANSP |
Academy of Natural Sciences of Philadelphia |
| MB |
Universidade de Lisboa, Museu Bocage |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Grammia allectans
| Schmidt, B. Christian 2009 |
Grammia allectans
| Ferguson DC & Opler PA 2006: 11 |
Grammia nevadensis (Grote & Robinson)
| Ferguson DC & Schmidt BC 2007: 40 |
| Ferguson DC & Opler PA 2006: 11 |
Grammia nevadensis geneura (Strecker)
| Ferguson DC & Schmidt BC 2007: 42 |
| Ferguson DC & Opler PA 2006: 11 |
Grammia nevadensis superba (Stretch)
| Ferguson DC & Schmidt BC 2007: 41 |
| Ferguson DC & Opler PA 2006: 11 |
Grammia nevadensis gibsoni (McDunnough)
| Ferguson DC & Schmidt BC 2007: 42 |
| Ferguson DC & Opler PA 2006: 11 |
Grammia nevadensis
| Ferguson DC & Opler PA & Smith MJ 2000: 48 |
Grammia nevadensis
| Ferguson DC & Opler PA & Smith MJ 2000: 48 |
Grammia nevadensis (Grote & Robinson)
| Grimble DG & Beckwith RC & Hammond PC 1992: 92 |
Grammia allectans
| Ferguson DC 1985: 231 |
Apantesis nevadensis (Grote & Robinson)
| Franclemont JG 1983: 117 |
Apantesis geneura superba (Stretch)
| Franclemont JG 1983: 117 |
Apantesis nevadensis (Grote & Robinson)
| Medlar WP 1940: 118 |
Callarctia nevadensis
| Smith ME 1938: 10 |
Apantesis gibsoni
| McDunnough J 1937: 152 |
Arctia nevadensis
| Coquillett DW 1898: 250 |
Arctia superba
| Strecker H 1880: 270 |
Arctia nevadensis
| Grote AR & Robinson CT 1866: 1 |