Grammia williamsii, (DODGE)
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2008.00496.x |
|
persistent identifier |
https://treatment.plazi.org/id/03B987FA-FF9A-F710-FCB4-6EE0FEE9E572 |
|
treatment provided by |
Felipe |
|
scientific name |
Grammia williamsii |
| status |
|
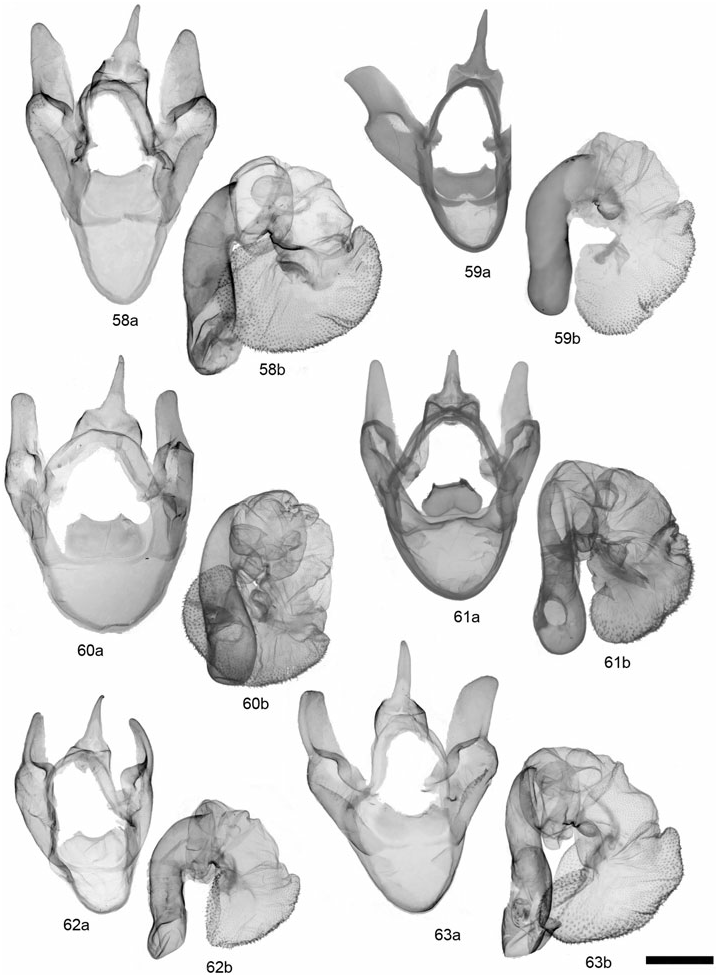
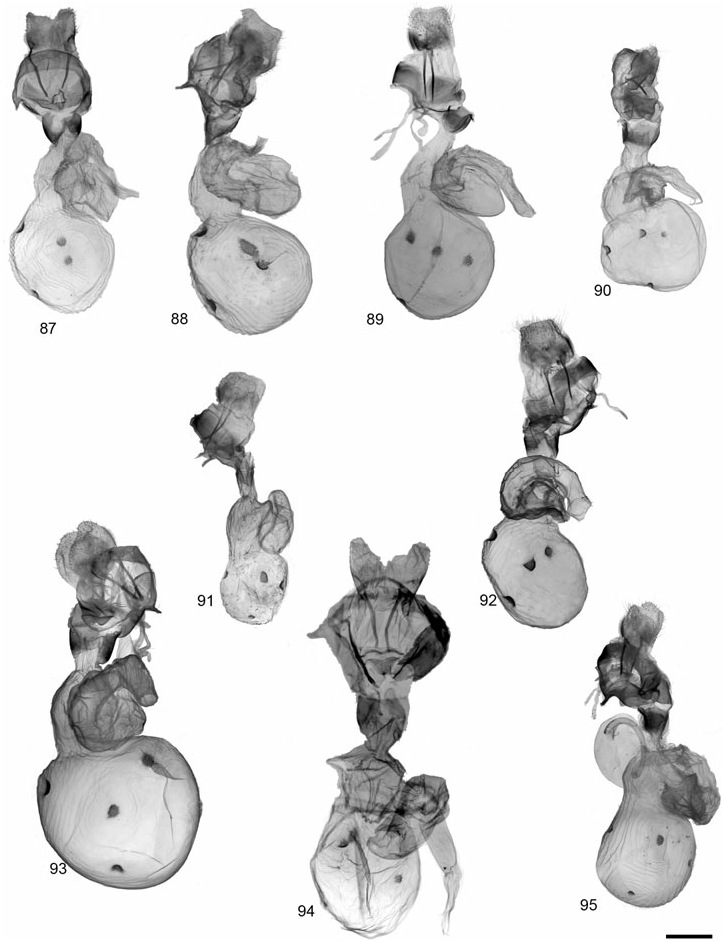
GRAMMIA WILLIAMSII (DODGE) ( FIGS 28, 62 View Figures 58–63 , 89 View Figures 87–95 , 121 View Figures 120–125 )
Arctia williamsii Dodge, 1871 ; Canadian Entomologist, 3: 167
Arctia determinata Neumögen, 1881b ; Papilio 1: 28 Apantesis tooele Barnes & McDunnough, 1910 ; Canadian Entomologist, 42: 208–209;
Apantesis tooele ab. ophir Barnes & McDunnough, 1910; Canadian Entomologist, 42: 208–209; Apantesis bolanderi ab. confluentis Strand, 1919; Lepidopterorum Catalogus, 22: 283. Revised synonymy.
Apantesis bolanderi confluentis Strand ; Franclemont, 1983: 117.
Apantesis williamsii (Dodge) ; Franclemont, 1983: 117. Apantesis williamsii tooele (Barnes & McDunnough) ; Franclemont, 1983: 117.
Grammia williamsii (Dodge) ; Ferguson & Opler, 2006: 10.
Grammia williamsii tooele (Barnes & McDunnough) ; Ferguson & Opler, 2006: 10.
Type material: Arctia williamsii : described based on a single female specimen from ‘ Colorado Territory’. The specimen is presumed lost or destroyed. An illustration is given in the original description, although according to Dodge (1871) himself, the illustration did not exactly match the specimen. However, the text description and illustration are consistent with the current concept of williamsii . In order to preserve the current usage of the name williamsii for this variable taxon, a male specimen bearing the following labels is hereby designated as neotype ( Fig. 28A): ‘leg. D.E. B[owman]. J[u]ne./ 15, 1990 Blacktail/ Creek Grand Co./ Colorado’, ‘ NEOTYPE / Arctia / williamsii Dodge / B.C. Schmidt, 2009’ [ CNC].
Arctia determinata : described based on an unstated number of specimens, collected by H.K. Morrison in 1877, and in 1880 by J. Doll and B. Neumögen, in ‘middle and southern Colorado’ ( Neumögen, 1881b). Two male syntypes are in the USNM, one labelled ‘Feb.8.81/ Arctia / determinata/ Neumögen/ Type.’, ‘Col./ B. Neumögen.’, ‘ Col.’, ‘TYPICUM/ SPECIMEN’ [examined], another with identical labels, in addition to the following: ‘ Arctiidae / genitalia slide/ No. AW1010’, ‘Type No. 33678/ U.S. N.M.’ [examined].
Apantesis tooele : described from six male syntypes from Provo and Eureka, UT. The male specimen in the USNM [examined] labelled ‘Provo Utah / VII-4–9/ Tom Spalding’, ‘ Apantesis / tooele/ B and McD/ Type ♂ ’, ‘ Arctiidae / genitalia slide/ No.AW1011’, ‘ LECTOTYPE / Apantesis tooele / Barnes and McD./ B.C. Schmidt, 2009′ is hereby designated as lectotype to ensure consistency in the application of the name to this taxon, and to avoid confusion with similar species.
Apantesis tooele ab. ophir: based on five male specimens of the form with entirely black thoracic vestiture; the name tooele has page priority. ophir was described as an aberrational form, and is therefore an unavailable infrasubspecific name.
Apantesis bolanderi ab. confluentis: Strand (1919) based this name on Hampson’s (1901: 405) concept of bolanderi . It is clear from Hampson’s description that he was referring to G. williamsii , and the ‘aberration’ he describes fits the description of G. williamsii with well-marked hindwings. In turn, Hampson’s concept of G. williamsii (as Apantesis viallimsi [sic]) fits the description of G. nevadensis , and not G. williamsii . Strand (1919) named confluentis based on Hampson’s Apantesis bolanderi Ab. 1 ( Hampson, 1901: 405–6).
Diagnosis: Grammia williamsii is one of the most common and widespread members of the genus, and can be difficult to recognize in parts of its range because of the variation in hindwing colour and hindwing dark markings. The reduced forewing banding pattern, straight (or nearly straight) postmedial line, and broad, ‘stubby-looking’ wings are diagnostic; curiously, G. williamsii males often also have a smaller abdomen relative to overall size compared to other Grammia . In the western USA, G. williamsii could be confused with G. bowmani , G. yavapai , or G. favorita , and additional diagnostic differences are given under these species. Other similar species include G. franconia and G. margo , from which it can be quickly be distinguished by the longer male antennal rami and later flight season of G. williamsii ; see ‘Diagnosis’ under G. margo and G. franconia for additional characters. In eastern Canada /north-eastern USA, some forms of G. figurata can look like G. williamsii , but G. figurata has a more rounded wing shape, and the distance between the medial and postmedial lines at the costa is greater in G. figurata . The ranges and habitats of G. williamsii and G. figurata are not known to overlap, with G. williamsii occurring in northern boreal forest habitats. See also G. bolanderi .
Description: Head – Vestiture of frons and vertex black, pale buff laterally; eyes well developed, diameter averaging slightly smaller in females than in males; (eyes strongly reduced in two females from CO and CA at high elevations); male antennae moderately bipectinate, rami averaging 4.66 ¥ 10 - 1 mm, 2.96¥ longer than intersegmental distance (N = 6); antenna with dark brown dorsal scales, occasionally with scattered buff scales; female antennae biserrate to slightly bipectinate. Thorax – Vestiture dark brown to black with broad, pale buff to pinkish buff borders on vertex, patagia, and tegulae; entirely dark thorax forms occur in subspecies tooele ; dark brown to black ventrally with yellowish to pinkish buff central tuft at base of forecoxae; legs predominantly dark brown with pale buff, varying to mostly pale buff. Abdomen – Dorsal ground colour pale pink to yellowish buff in males, yellow to pinkish orange in females, pale buff near apex; medial and lateral markings dark brown to black; pale buff ventrally, dark brown to black markings consisting of two rows of broad spots, sometimes broad and converging medially; females entirely dark brown ventrally. Forewing – Mean forewing length 14.9 mm (N = 6); ground colour chocolate brown to dark brown, discal cell area often darker; banding pattern reduced, basal band absent, antemedial usually absent or reduced to costal spot; medial band usually present, extending to but not beyond postcubital stripe; postmedial nearly always present, straight near costa and with little or no change in angle at M 3 vein; band colour pale to pinkish buff; fringe variable in colour, dark brown to pale buff; costa entirely dark brown or with variable amount of thin, pale buff border; anal margin usually white at least halfway to anal angle; anal dash absent in> 95% of specimens; underside similar to upperside, but bands less contrasting; sexes similar. Hindwing – Ground colour varying from dull orange-buff to pinkish buff, rarely yellowish, most commonly dull pink; brighter yellowish to pinkish orange in females; markings dark brown to black, well developed and often confluent, particularly postmedial and subterminal markings; antemedial markings well developed, often streaked towards wing base; ventrally with similar pattern and duller ground colour; females with reduced hindwing spots and brighter, often orange-pink, ground colour. Male genitalia – Distal portion of valve gradually tapering to rounded apex; clasper moderately developed, median ridge moderately developed; uncus broad-based, process evenly tapered to point; juxta wider than long; aedeagus with dorsad curve at 2/3 distance beyond base; distal chamber of vesica nearly globose, slightly kidney-shaped, scobinate, spinules relatively large; medial chamber roughly as wide as distal chamber. Female genitalia – Ductus bursae unsclerotized; corpus bursae more or less globose and relatively small, 2.5¥ width of ostium bursae; signa equal, round and relatively small (2.56 ¥ 10 - 1 mm); appendix bursae with broad, angled elbow, not evenly tapered; posterior apophysis 1.5¥ longer than length of papillae anales.
Biology: Collection dates of adults range from mid May to late August, depending on altitude and habitat. Most records from the Great Plains are from June, whereas boreal and mountain populations peak in July. Although this species is often the most common Grammia , I have seen few females, all reared, and they are not attracted to light, or are unable to fly to light when gravid. Larvae probably feed on many dicots and occasionally grasses; wild caught larvae have been recorded on Thermopsis rhombifolia (Fabaceae) , Senecio (Asteraceae) , Delphinium bicolor (Ranunculaceae) and Triticum aestivum (Poaceae) ( Byers, 1988, pers. observ.). Widely distributed, and found in montane and prairie grasslands, dry subalpine meadows, and dry (often sandy) boreal forest.
Distribution: Occurs from the NT east to the northern Great Lakes region, NB, and New England. Occurs throughout the northern Great Plains, and south at higher elevations to AZ and NM, west to southeastern BC and eastern CA ( Fig. 121 View Figures 120–125 ).
Molecular variation: Grammia williamsii exhibits a phenomenal range of geographically structured variation in cox 1 haplotypes, representative of two divergent mtDNA lineages within the genus ( Fig. 133 View Figure 133 ). Forty-nine specimens representing populations from all geographical regions within the species’ range showed 23 haplotypes, with a mean divergence of 2.5%, up to divergences of 6.5% ( Tables 2 and 3). The six specimens of G. williamsii tooele from two localities exhibited two unique haplotypes (WI12, WI13), surprisingly divergent from G. williamsii , and indeed all other Grammia ( Fig. 134 View Figure 134 ), with the most similar haplotype about 3% divergent ( Fig. 134 View Figure 134 ). North-eastern NV individuals of G. williamsii , geographically adjacent to the G. w. tooele sample sites, had a haplotype (WI14) more similar to other G. williamsii haplotypes in the Western lineage ( Fig. 134 View Figure 134 ).
Remarks: This species is often the most abundant Grammia in the western parts of its range. Although it is also one of the most widespread species, it expresses relatively little geographically structured phenotypic variation, and often considerable variation within a population. In long series, boreal and mountain populations tend to have a pinker hindwing with suffused and expanded dark markings, compared to the Great Plains populations which show a reduction in the hindwing markings with a duller, often orangish ground colour and slightly larger wing span.
GRAMMIA WILLIAMSII WILLIAMSII (DODGE)
( FIG. 28A–D)
Diagnosis: Nominate G. williamsii shows relatively little geographical variation, but see ‘Remarks’ under G. williamsii above. See Diagnosis under G. williamsii tooele for comparison to this subspecies.
Distribution: The range of this subspecies is here considered to include the majority of the species’ range as given under G. williamsii , with the exception of central UT, where tooele occurs.
GRAMMIA WILLIAMSII TOOELE (BARNES & MCDUNNOUGH) ( FIG. 28E)
Diagnosis: This subspecies is distinguished from nominal williamsii by the occurrence of the dark thorax form, reduced hindwing dark markings, slightly larger size, and brighter orange-pink hindwing ground colour.
Distribution: Central UT, from Cache county south to Beaver county.
Remarks: Grammia williamsii tooele was originally described as a species distinct from williamsii , distinguished by the black thorax, brighter hindwing colour, and larger size compared to williamsii . Barnes & McDunnough (1918) subsequently relegated tooele to subspecies status of williamsii , and the status of tooele has subsequently remained unchanged. The absence of shared haplotypes with, and significant cox 1 sequence divergence from G. williamsii suggest that G. w. tooele could be distinct species, but I am unable to find consistent morphological differences separating the two taxa in UT, with significant overlap in size and hindwing coloration. Specimens from some populations exhibit variation from tooele to williamsii phenotypes, although the absence of tooele phenotypes in eastern NV is noteworthy. It is possible that the dark-thorax form is a result of past or ongoing hybridization with G. nevadensis nevadensis , supported by the fact that NV samples of G. w. williamsii displayed haplotypes virtually identical to two G. n. nevadensis haplotypes from UT and southeastern OR ( Fig. 134 View Figure 134 ). Conversely, this does not explain the molecular divergence between UT G. w. tooele and G. w. williamsii from adjacent parts of NV ( Fig. 134 View Figure 134 ). Based on the apparent intergradation of tooele and williamsii phenotypes, geographically restricted occurrence of the dark thorax (nominate tooele ), and the distinct cox 1 lineage, I treat tooele as a subspecies. Extensive sampling of key geographical areas would shed some light on this interesting situation, namely where tooele and williamsii should overlap or intergrade in western and central/northcentral UT.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Grammia williamsii
| Schmidt, B. Christian 2009 |
Grammia williamsii (Dodge)
| Ferguson DC & Opler PA 2006: 10 |
Grammia williamsii tooele (Barnes & McDunnough)
| Ferguson DC & Opler PA 2006: 10 |
Apantesis bolanderi confluentis
| Franclemont JG 1983: 117 |
Apantesis williamsii (Dodge)
| Franclemont JG 1983: 117 |
| Franclemont JG 1983: 117 |