Teruelius, Lowe & Kovařík, 2019
|
publication ID |
https://doi.org/10.18590/euscorpius.2019.vol2019.iss281.1 |
|
publication LSID |
lsid:zoobank.org:pub:FEBA0106-02A3-4465-8D39-9FF32634EEF |
|
DOI |
https://doi.org/10.5281/zenodo.7143624 |
|
persistent identifier |
https://treatment.plazi.org/id/54CB8128-BCFD-4B1F-A947-153C7CDD5B83 |
|
taxon LSID |
lsid:zoobank.org:act:54CB8128-BCFD-4B1F-A947-153C7CDD5B83 |
|
treatment provided by |
Felipe |
|
scientific name |
Teruelius |
| status |
gen. nov. |
Genus Teruelius View in CoL gen. n.
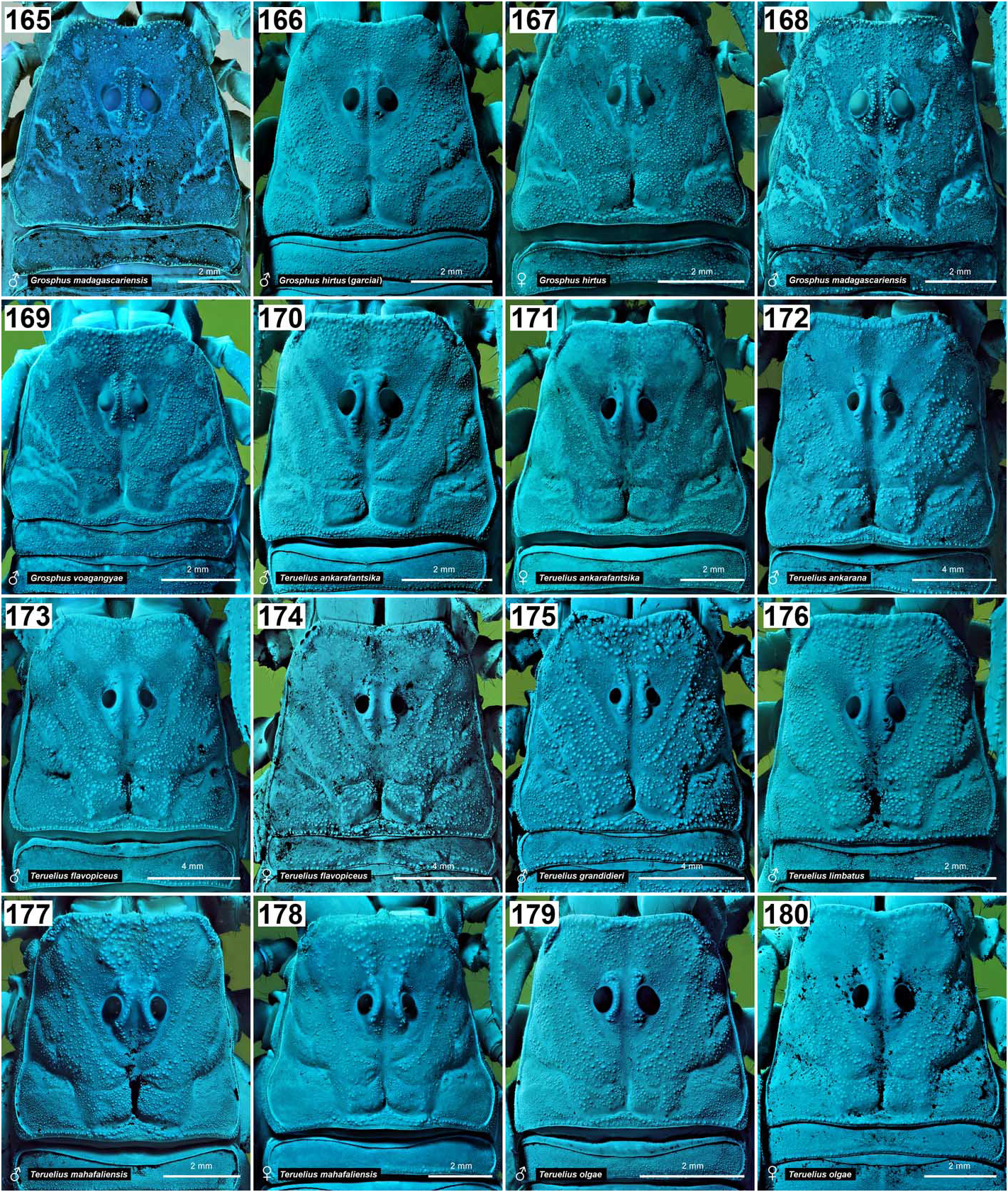
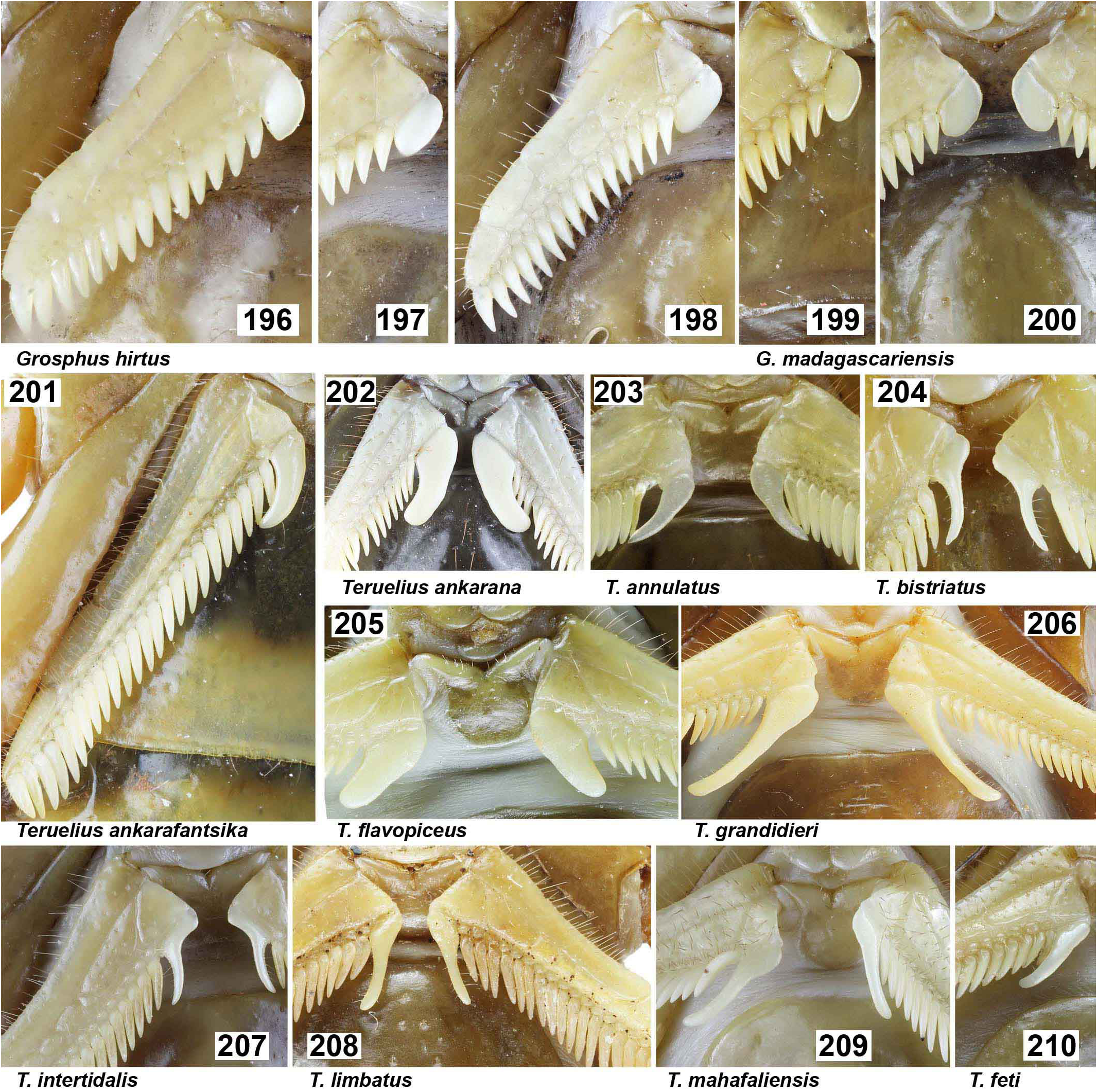
( Figs. 5–8 View Figures 5–8 , 13–20 View Figures 9–20 , 23–39 View Figures 21–27 View Figures 28–29 View Figures 30–35 View Figures 36–39 , 44–51 View Figures 40–51 , 71–85 View Figures 71–85 , 90–93 View Figures 90–93 , 99–105, 106–121, 137–144 View Figures 133–144 , 150–160 View Figures 145–157 View Figures 158-160 , 170–180 View Figures 165–180 , 186–195 View Figures 181–195 , 201–210 View Figures 196–210 , 216–226 View Figures 211–226 , 229–230 View Figures 227–230 , 235–238 View Figures 231–238 , 387–579 View Figures 387–390 View Figures 391–408 View Figures 409–417 View Figures 418–419 View Figures 420–421 View Figures 422–425 View Figures 426–435 View Figures 436–438 View Figures 439–446 View Figures 447–458 View Figure 459 View Figures 460–472 View Figures 473–476 View Figures 477–490 View Figures 491–494 View Figures 495–499 View Figures 500–511 View Figures 512–515 View Figures 516–521 View Figures 522–525 View Figures 526–537 View Figures 538–541 View Figures 542–546 View Figures 547–551 View Figures 552–563 View Figures 564–575 View Figures 576–579 , 584–605 View Figures 584–587 View Figures 588–589 View Figures 590–592 View Figures 593–594 View Figures 595–596 View Figures 597–599 View Figures 600–601 View Figures 602–603 View Figures 604–605 , Tabs. 1–4 View Table 1 )
http://zoobank.org/urn:lsid:zoobank.org:act:54CB8128- BCFD-4B1F-A947-153C7CDD5B83
Grosphus Vachon, 1940: 254 View in CoL , 256; Lourenço et al., 2007b: 375; Prendini & Esposito, 2010: 675–676; Loria & Prendini, 2014: 25; Loria & Prendini, 2018: 184.
TYPE SPECIES. Buthus limbatus Pocock, 1889 .
ETYMOLOGY. The generic epithet Teruelius (masculine) is a patronym honoring Rolando Teruel from Cuba in recognition of his many important contributions to the knowledge of scorpions.
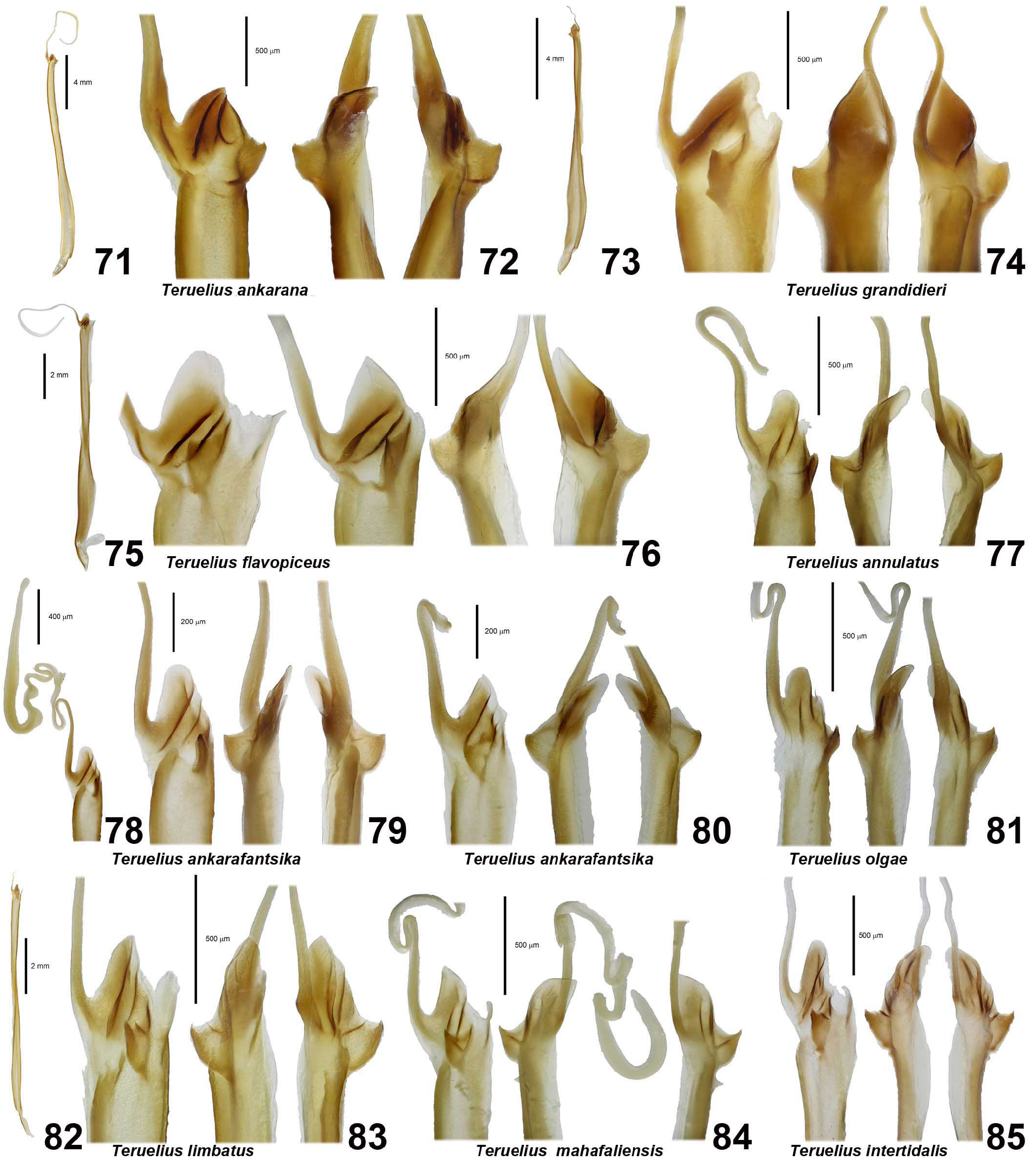
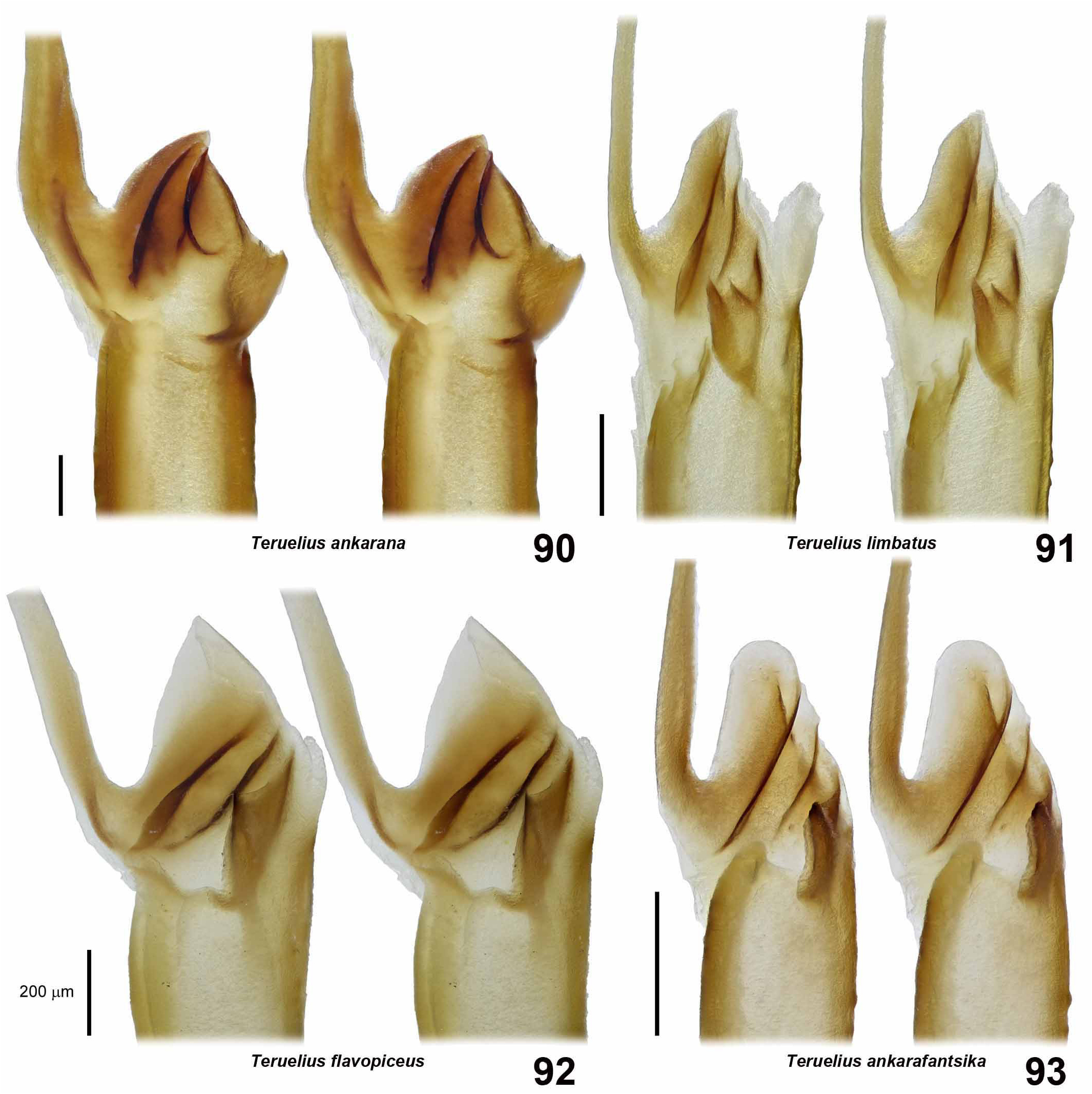
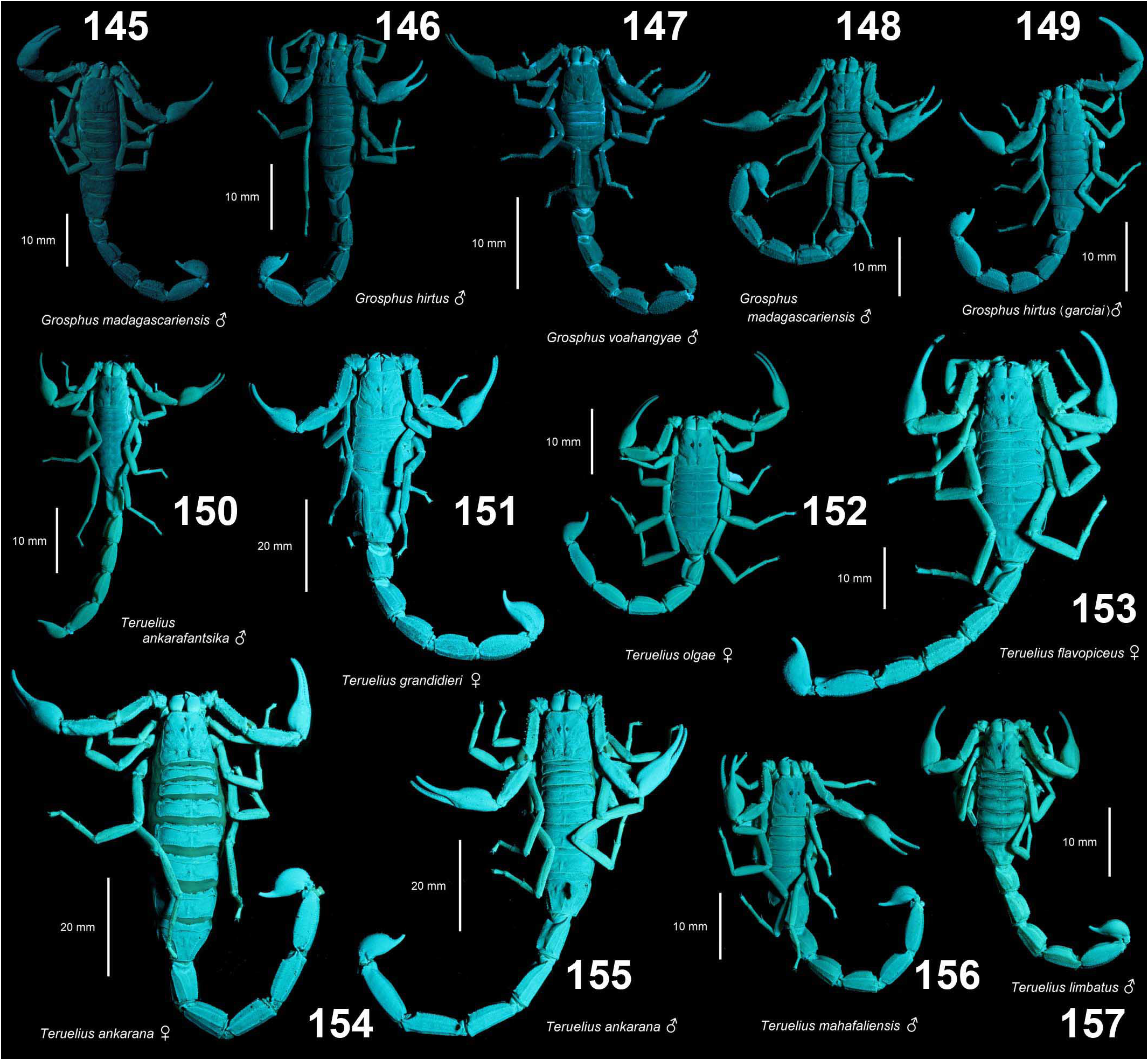
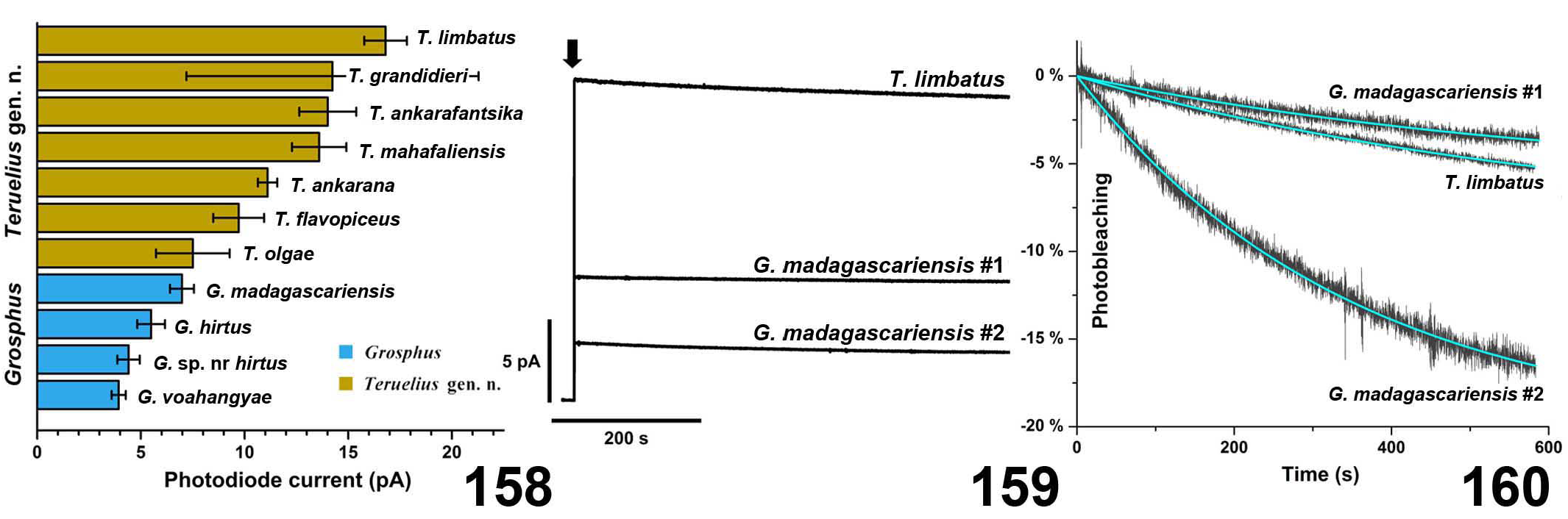
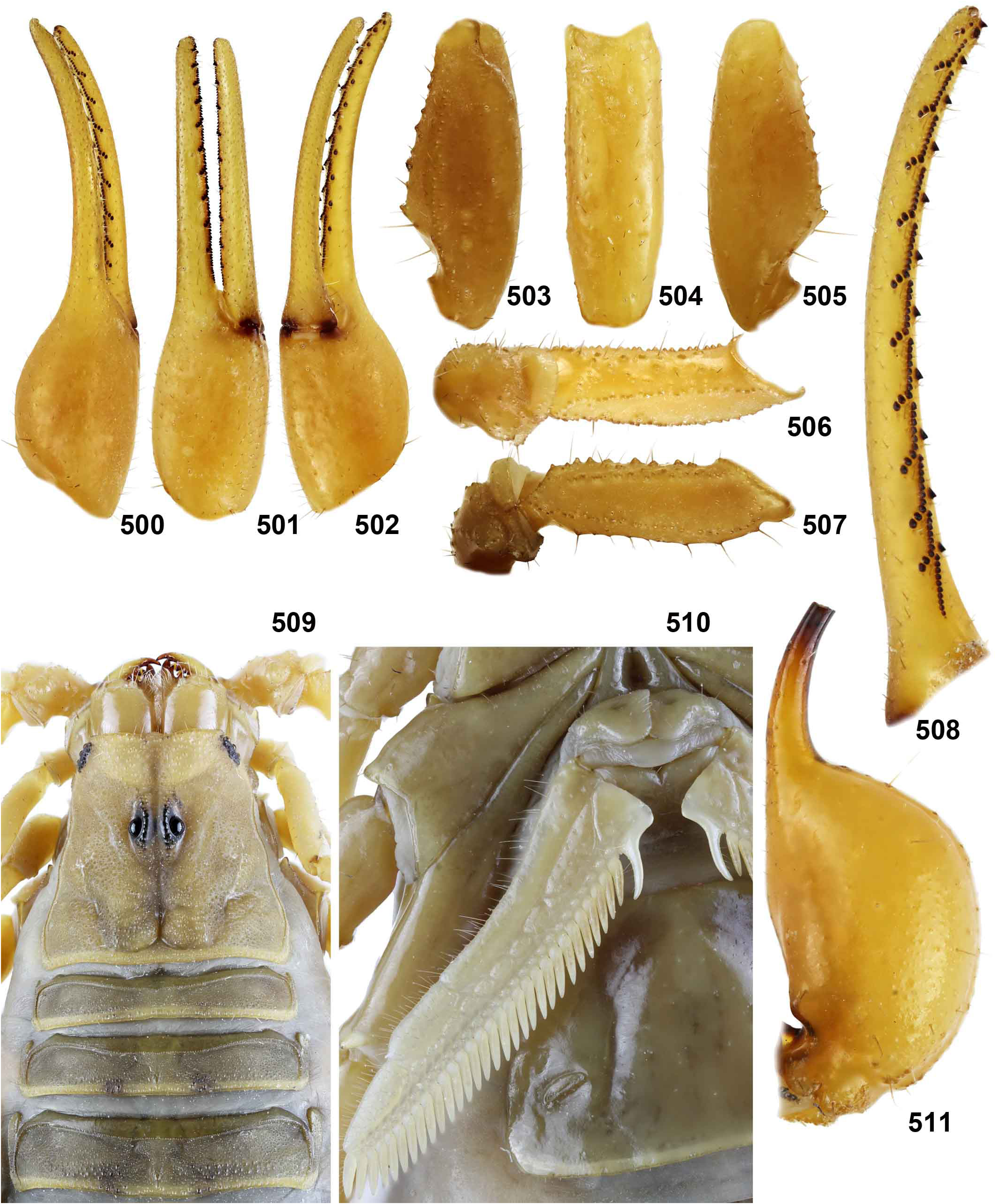
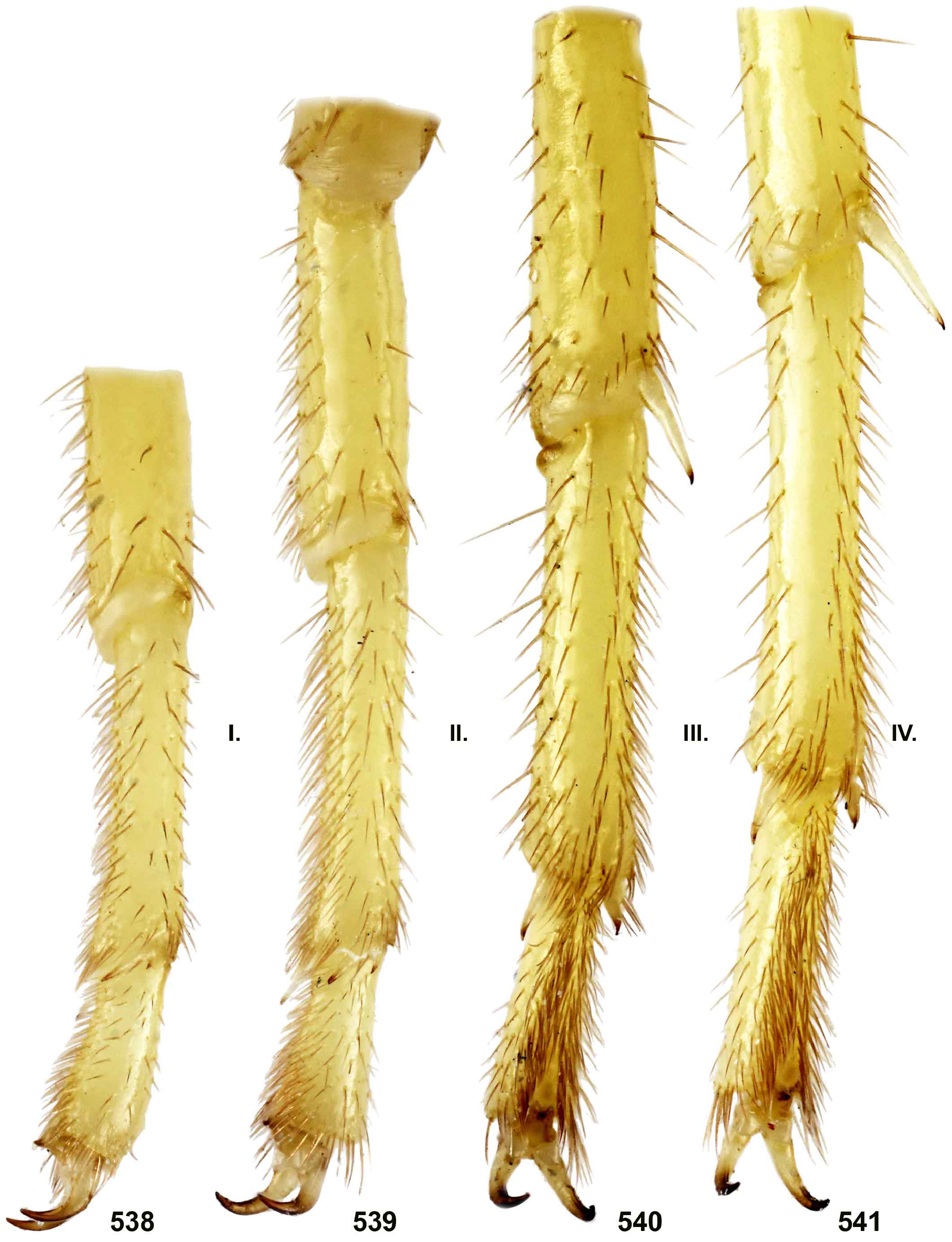
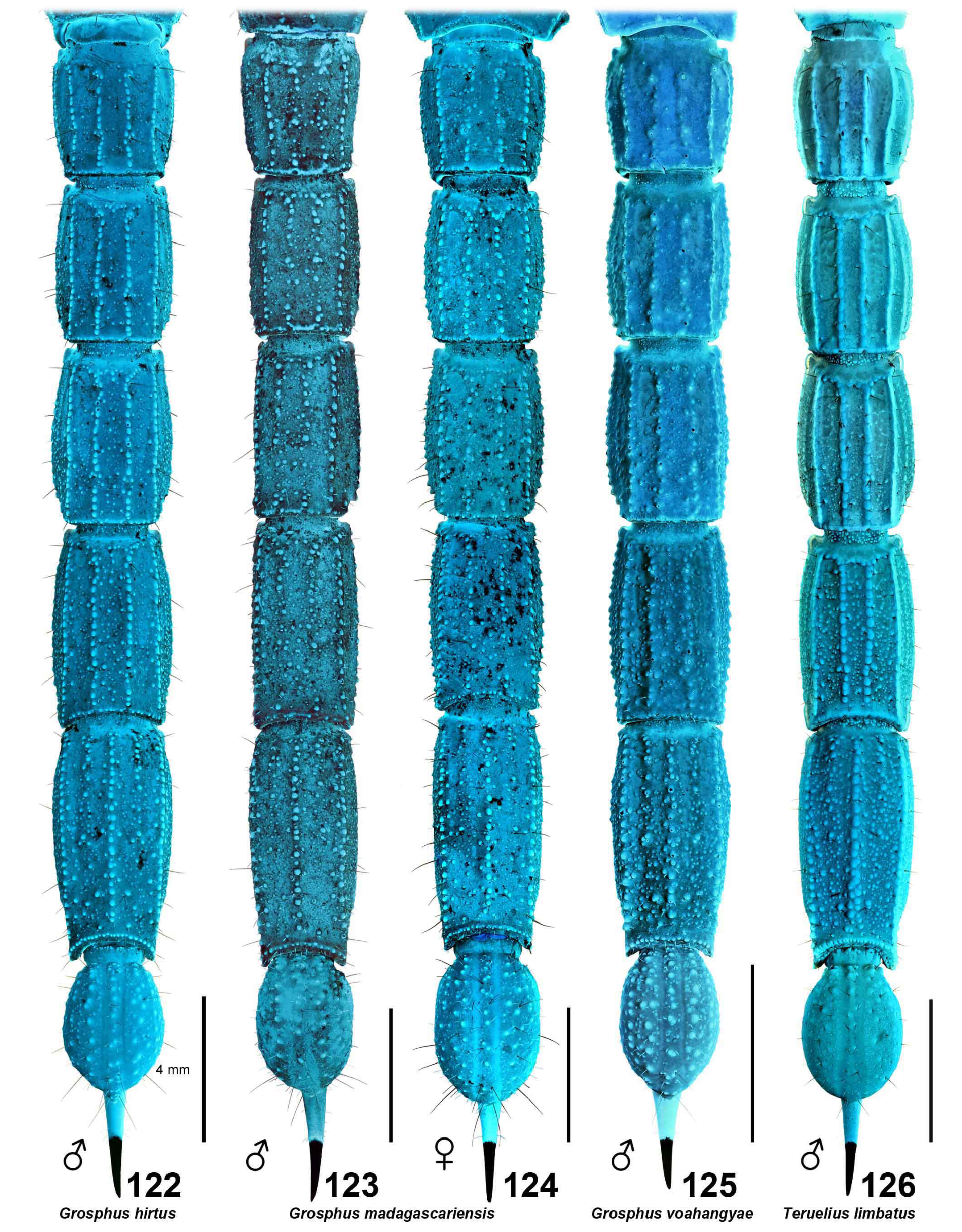
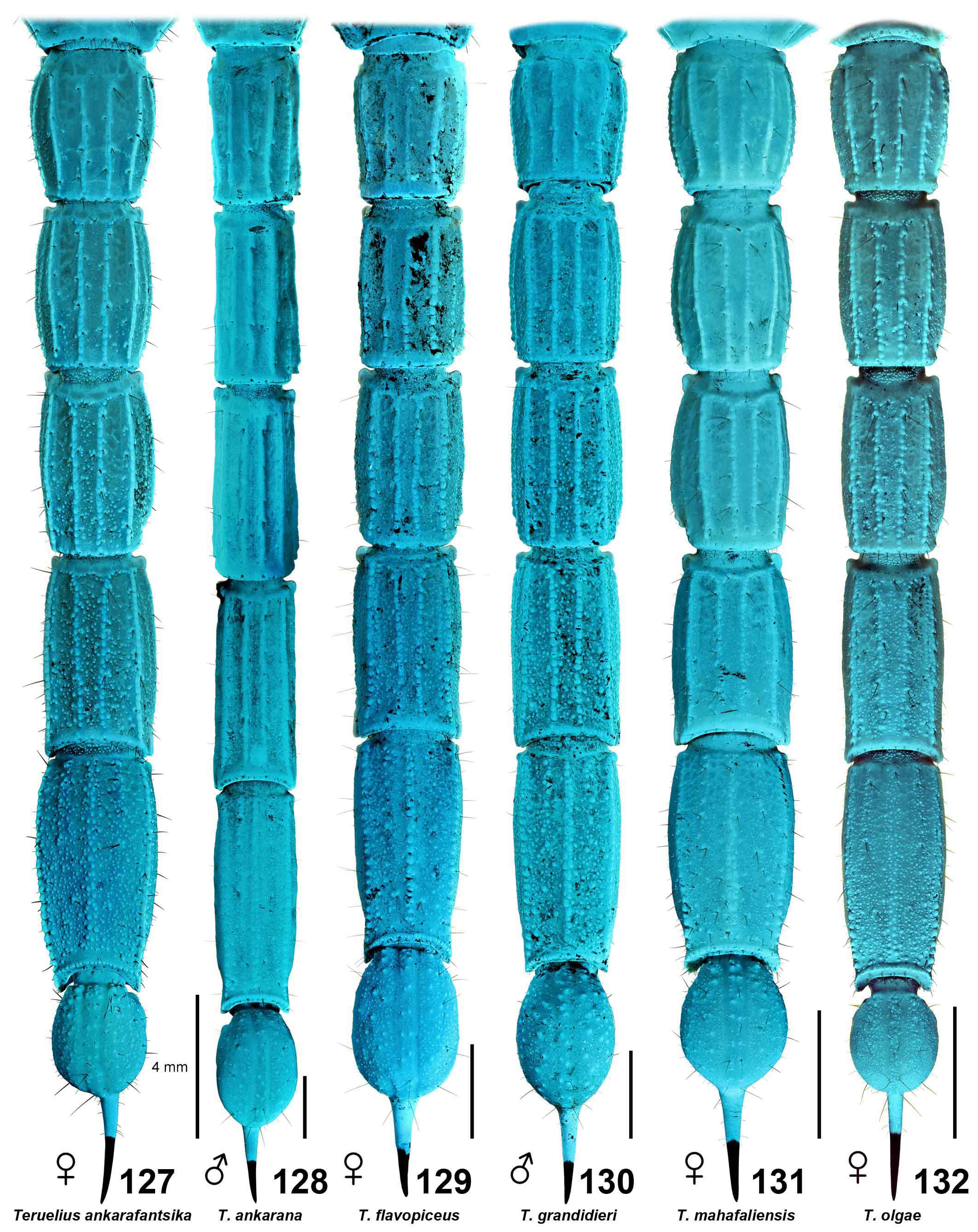
DIAGNOSIS. A member of the ‘ Grosphus ’ group differentiated as follows: medium-sized to large-sized scorpions, adults ca. 35–120 mm in length; pedipalp finger granule rows 10–15 ( Figs. 402 View Figures 391–408 , 431 View Figures 426–435 , 452 View Figures 447–458 , 485–486 View Figures 477–490 , 508 View Figures 500–511 , 521 View Figures 516–521 , 529 View Figures 526–537 , 560 View Figures 552–563 ), movable finger typically with 4 external subdistal granules; femur trichobothrium d 2 straddling dorsointernal carina, or located on dorsal surface ( Figs. 13–20 View Figures 9–20 ); chela manus with petite trichobothrium Eb 3 near Eb 2, closer than half the distance between Eb 1 and Eb 2 ( Figs. 23–24 View Figures 21–27 ); manus trichobothrium V 2 roughly collinear with V 1 along chela axis or slightly displaced internally; higher pectinal tooth counts: ♂ 25– 41, ♀ 24–35 ( Figs. 28–31 View Figures 28–29 View Figures 30–35 ); basal pectinal tooth of females wide, with elongate, tapering distal extension, distinctly longer than other teeth ( Figs. 44–51 View Figures 40–51 , 201–210 View Figures 196–210 , 411 View Figures 409–417 , 510 View Figures 500–511 , 526 View Figures 526–537 ); hemispermatophore capsule short, carinate, posterior lobe rounded, without lanceolate extension ( Figs. 71–85 View Figures 71–85 ); sternites with narrow, slit-like spiracles ( Figs. 99–105); metasoma I with ventromedian carinae moderately to weakly crenulate or smooth to obsolete ( Figs. 126–132 View Figures 122–126 View Figures 127–132 ); telson with oval or bulbous vesicle, without subaculear tubercle in adults ( Figs. 186–195 View Figures 181–195 ); legs with ventral surface of telotarsus densely setose or scopulate, with broad, brush-like strips of> 20 long filiform macrosetae ( Figs. 138–144 View Figures 133–144 , 216–226 View Figures 211–226 , 409–417 View Figures 409–417 , 487–490 View Figures 477–490 , 512–515 View Figures 512–515 , 538–541 View Figures 538–541 , 576–579 View Figures 576–579 ); telotarsus with dorsal terminal process of normal size; cuticle with strong UV fluorescence ( Figs. 150–157 View Figures 145–157 ).
SUBORDINATE TAXA.
Teruelius ankarafantsika ( Lourenço, 2003) comb. n. Teruelius ankarana ( Lourenço & Goodman, 2003) comb. n. Teruelius annulatus ( Fage, 1929) comb. n.
Teruelius bemaraha ( Lourenço, Wilmé & Waeber, 2018) comb. n.
Teruelius bicolor ( Lourenço, 2012) comb. n.
Teruelius bistriatus ( Kraepelin, 1900) comb. n.
Teruelius eliseanneae ( Lourenço & Wilmé, 2016) comb. n. Teruelius feti ( Lourenço, 1996) comb. n.
Teruelius flavopiceus ( Kraepelin, 1900) comb. n.
Teruelius ganzhorni ( Lourenço, Wilmé & Waeber, 2016) comb. n.
Teruelius grandidieri ( Kraepelin, 1900) comb. n.
Teruelius intertidalis ( Lourenço, 1999) comb. n.
Teruelius limbatus ( Pocock, 1889) comb. n.
Teruelius magalieae ( Lourenço, 2014) comb. n.
(= T. mahafaliensis ?)
Teruelius mahafaliensis ( Lourenço, Goodman & Ramilijaona, 2004) comb. n.
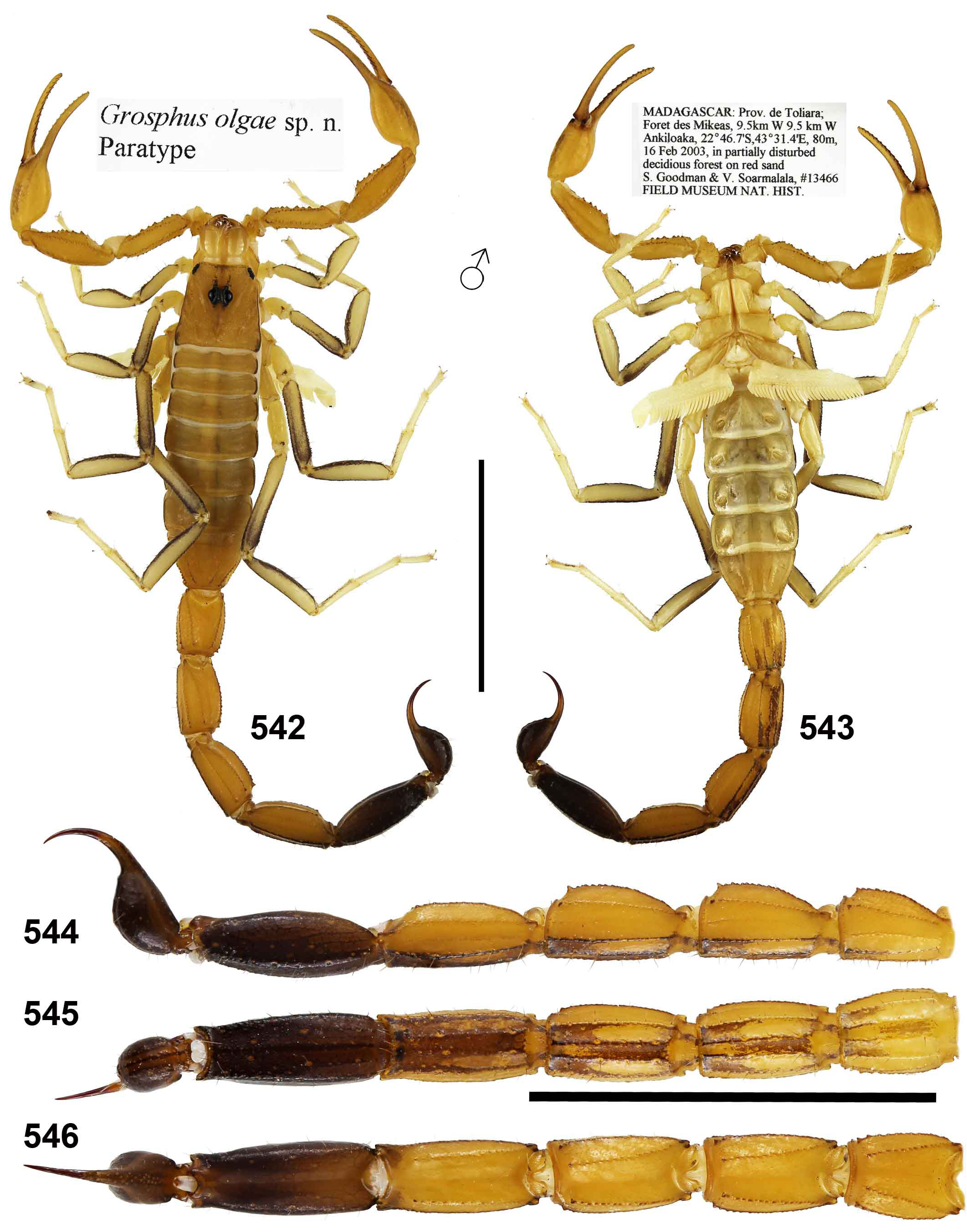
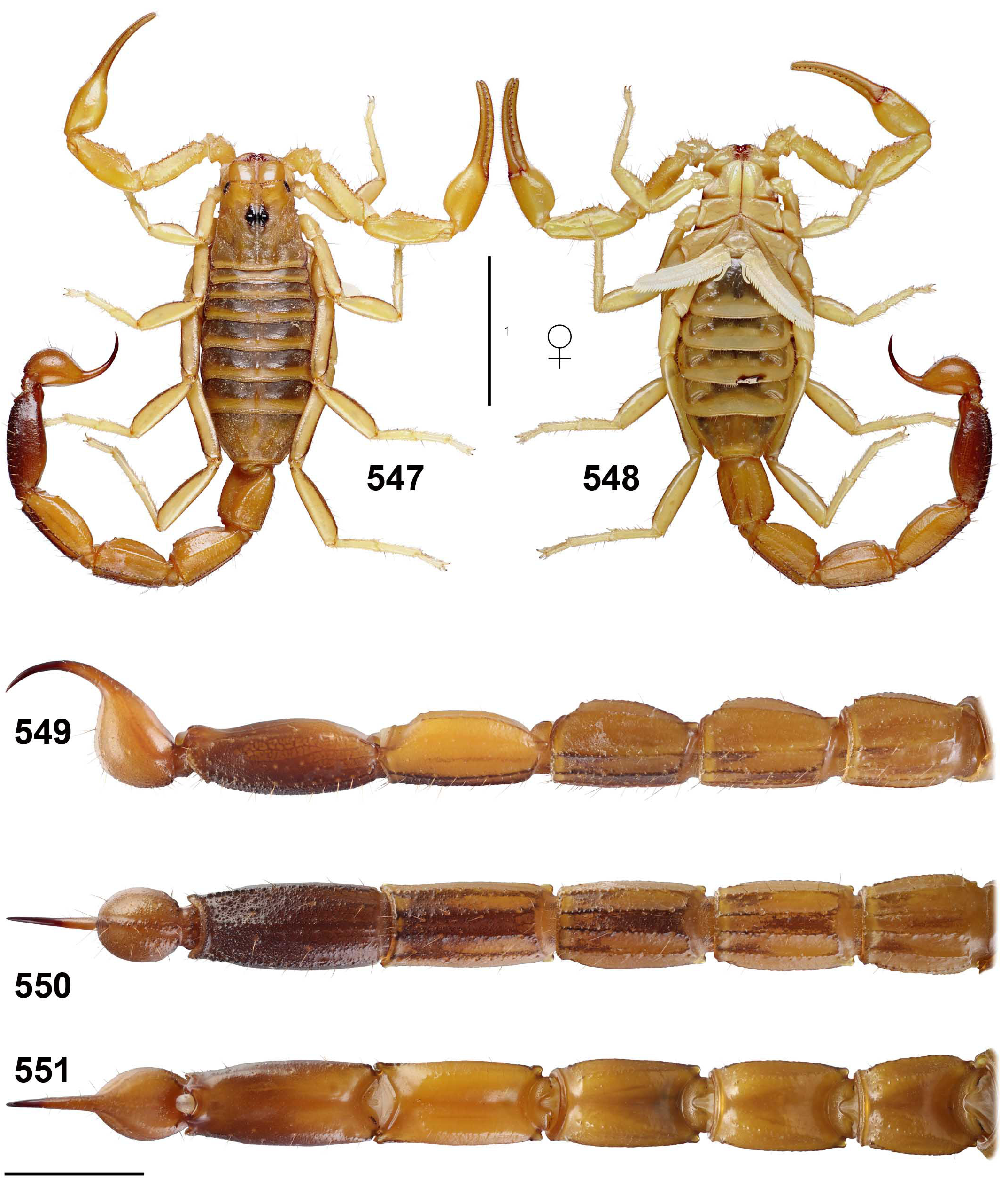
Teruelius olgae ( Lourenço, 2004) comb. n.
Teruelius sabineae ( Lourenço & Wilmé, 2016) comb. n. Teruelius waeberi ( Lourenço & Wilmé, 2016) comb. n.
See Tables 1–3 View Table 1 for diagnostic characters used to place the above species under Teruelius gen. n.
REMARKS. Recognition of Teruelius gen. n. as a separate genus, distinct from Grosphus , necessitates revision of some previous concepts about taxonomy and biogeography of Grosphus . Lourenço et al. (2017) associated G. ‘ simoni ’(= G. madagascariensis ) with G. rakotoariveloi and G. ‘ halleuxi ’, and subsequently Lourenço et al. (2018b) elaborated on a ‘ Grosphus simoni ’ group, treating it as a monophyletic unit of closely related “sister” species including the aforementioned three, plus G. ambre , G. bemaraha and G. mahafaliensis . A group diagnosis was not provided but, for G. bemaraha , mention was made of “a number of features such as spiniform granules on the dorsal carinae of metasomal segments II-IV and on internal carinae of pedipalp femur and patella”. We place G. bemaraha under Teruelius gen n., on the basis of narrow spiracles, dense tarsal setation and high pectinal tooth count ( Table 3 View Table 3 ), and consider spiniform granules shared with G. simoni to be a homoplasy. G. bemaraha was claimed to be closer to G. rakotoariveloi , but these species belong to different genera. Lourenço et al. (2018b: 74, fig.1) included G. mahafaliensis in the ‘ simoni ’group, perhaps due to similarities to G. bemaraha , noting in particular a high number of pectine teeth (but it lacks spiniform granules on metasomal carinae). In contrast, we find that T. mahafaliensis comb. n. is very far removed from G. ‘ simoni ’ (= G. madagascariensis ), differing in all nine genus-level diagnostic characters, and in the fundamental architecture of the hemispermatophore capsule (cf. Fig. 84 View Figures 71–85 vs. Figs. 52–57 View Figures 52–70 ).
Lourenço et al. (2018b) discussed biogeographic hypotheses attempting to explain the distribution of the incongruous, polyphyletic ‘ Grosphus simoni ’ group. The very wide distribution of the group meant that it was “adapted to humid, dry and subarid environments”, and the two most disjunct species, G. bemaraha and G. mahafaliensis from western and southern localities, were speculated to “belong to relict populations, which may have survived in humid refugia encountered in the sedimentary basins during the dry episodes of the paleoclimate oscillations”. However, these two species are not closely related to the other four group members, but belong to Teruelius gen. n., whose ancestors may have already been adapted to dry environments. Although Pleistocene climatic fluctuations could be relevant for recent speciation events in Grosphus and Teruelius gen. n., the many correlated characters separating these two genera suggest a far earlier split, as in other taxa. For example, dated molecular phylogenies of Madagascar archaeid spiders (Wood et al., 2015), Brookesia and other chameleons ( Tolley et al., 2013; Townsend et al., 2009) and Zonosaurus plated lizards ( Blair et al., 2015) have revealed that the majority of divergences in these other endemic taxa are quite deep, occurring long before the advent of Pleistocene climate cycles.
Species of Teruelius gen. n. can be loosely subdivided by size and coloration: large species, T. flavopiceus , T. ankarana , T. grandidieri and T. bicolor ; species with patterns of dark stripes on tergites (‘ bistriatus ’ group of Lourenço & Wilmé, 2016): T. ankarafantsika , T. bistriatus , T. eliseanneae , T. feti , T. limbatus , T. sabineae and T. waeberi ; species with almost uniform yellow, orange or brown tergites, and maybe darker metasoma IV or V: T. annulatus , T. bemaraha , T. ganzhorni , T. intertidalis , T. magalieae , T. mahafaliensis and T. olgae . These groupings have been used to construct species keys, in conjunction with some other characters including shapes of female basal pectine teeth ( Fage, 1929; Lourenço, 2003c, 2004a, 2014; Lourenço et al., 2007b; Vachon, 1969). Monophyly of these groupings remains to be tested.
NEW SYNONYMIES.
Grosphus makay Lourenço & Wilmé, 2015 = Teruelius feti ( Lourenço, 1996) comb. n., syn. n.
Grosphus feti was described by Lourenço (1996b) from a juvenile male holotype, ostensibly collected from “Prov. Tulear, Tanjon’ I Vohimena [= Cap Sainte Marie] Réserve spéciale, X.1995 ” and deposited in FMNH. We loaned and studied the holotype and a second juvenile male labeled as “ ♂ paratype ” (which we concur is conspecific with the holotype). Until now, this species was only known from these two types. Associated GoogleMaps with the type, we found locality labels ( Fig. 459 View Figure 459 ) that differ from the published type locality: “ MADAGASCAR: Province de Toliara, Fôret de Vohimena, 35 km SE Sakaraha, 17-24.i.1996, MyrCE-7541, 22°41.0’S 44°49.8’E, 780 m, S. M. Goodman 0000 011 031 FMNH- INS Grosphus feti Lourenço HOLOTYPE: det. 1996 ”. This locality is ca. 325 km roughly north of the published type locality at Cap Sainte Marie and the collection date of January 1996 is several months later. Either a labeling error occurred after description, or the published type locality is incorrect. Cap Sainte Marie is a biological study area and frequent source of scorpion materials (e.g., Lourenço & Wilmé, 2016) so data labels of specimens could have been confused.
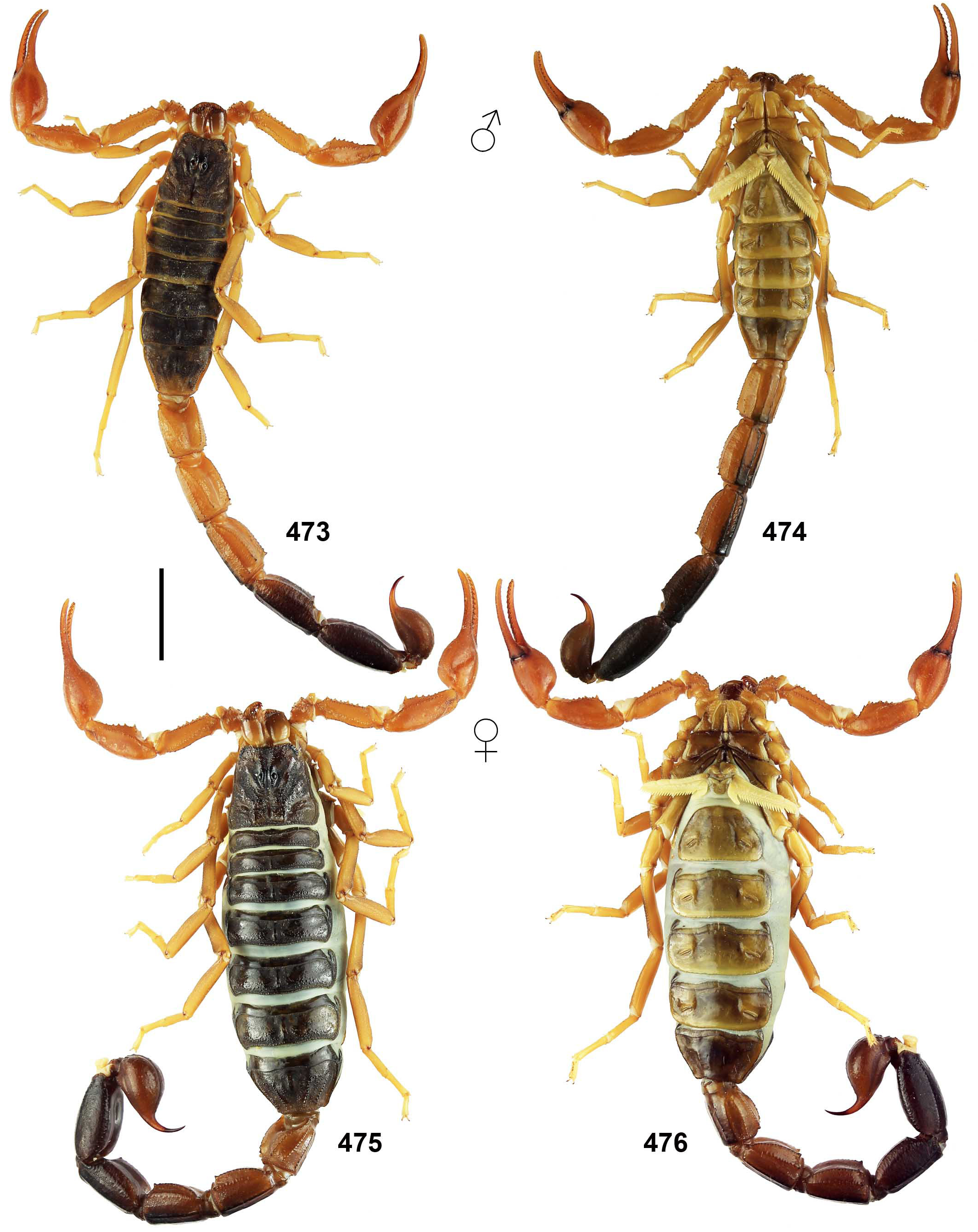
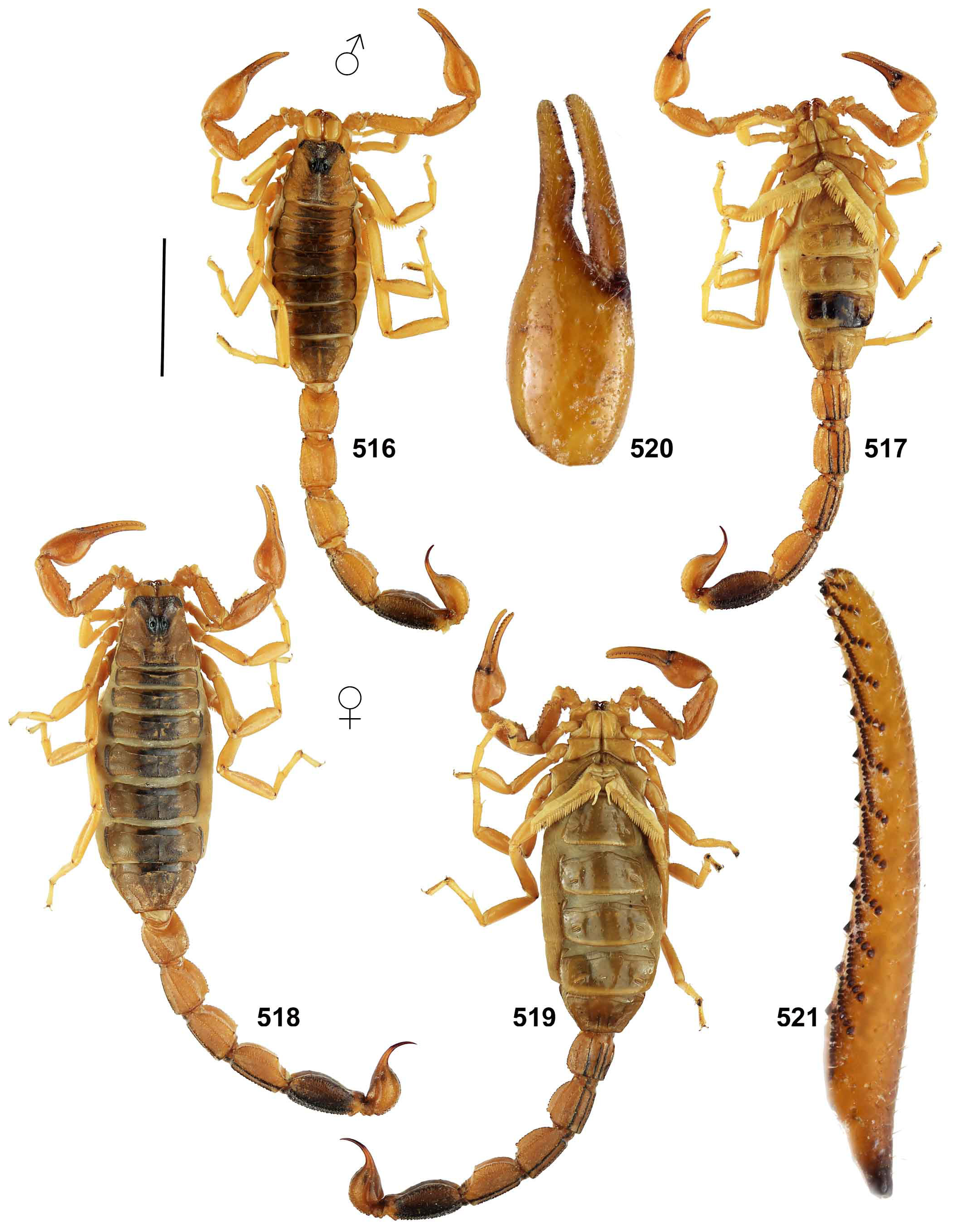
The FMNH label site is ca. 145 km south and slightly west of the type locality of Grosphus makay ( Lourenço & Wilmé, 2015b) : “Region Atsimo-Andrefana, ex Province of Toliara, Makay Mts., General Collection, dry-Forest on sandy soil, 12/III/2010 (B. L. Fisher et al.). BLF25549. Female holotype (CAS)”. These two localities have sandstone substrates, similar elevation and are located in the same general bioclimatic region. Comparison of the published habitus of the holotype adult female of T. makay and the holotype juvenile male of T. feti revealed very similar morphology and morphometrics even though sex and age differ. Most notably, their color patterns are identical in all details including: pattern of fuscosity on interocular triangle of carapace with pale cut-out behind lateral eyes; thin median line, precise fuscous banding patterns and transverse lateral striping on all tergites; darkly marked ventrolateral and ventromedian carinae on metasoma I–IV; fuscous patterns on metasoma V and telson; short fuscous strip on interno-proximal margin of pedipalp patella; leg femora with distal short, pale cut-outs on distal dark areas of prolateral surfaces and pale narrow lines on dorsal margins; and leg patellae with fuscous ventral margins on prolateral surfaces (compare Fig. 459 View Figure 459 to fig. 13 of Lourenço & Wilmé, 2015b). These very particular details of pigmentation pattern are not found in T. limbatus which was regarded as a closely related species ( Figs. 5–8 View Figures 5–8 , 516–521 View Figures 516–521 ). We also examined two adult females, near topotypes of G. makay from the Makay Mountains , that exhibited the same coloration patterns and morphometrics as T. feti ( Fig. 459a View Figure 459 ). We allowed for the fact that juveniles of pigmented scorpions usually display darker, more intense markings, and that in adults these color patterns are somewhat faded. Details of coloration pattern have been given high priority as characters for species-level taxonomy of Grosphus ( Lourenço et al., 2009b; Lourenço, 2014). We therefore consider Grosphus makay to be a junior synonym of T. feti , and the correct type locality of the latter to be that indicated on FMNH data labels. T. feti was never again collected from Cap Sainte Marie in over two decades of fieldwork since its description, although other scorpion species (e.g., T. sabineae ) were discovered there. The adult male remains unknown. Our opinion could be verified by collection and analysis of topotypic adult and juvenile specimens from the FMNH locality. The description of T. feti ( Lourenço, 1996b: 14) noted the juvenile status of the holotype which is ca. 30 mm, but Lourenço (2014: 636) diagnosed the species as “of small size with a total length of 30 to 40 mm ”. Small size cannot be a species diagnostic character if it is a property of the juvenile, and adult males are probably medium-sized, comparable to an adult female of G. makay , ca. 56 mm in body length. Our synonymy also implies that this is not a microendemic species of the upper Central Menabe, as suggested of Lourenço & Wilmé (2015b). The T. feti emended type locality lies a significant distance south of the Makay mountains , in the Mangoky watershed ( Wilmé et al., 2006).
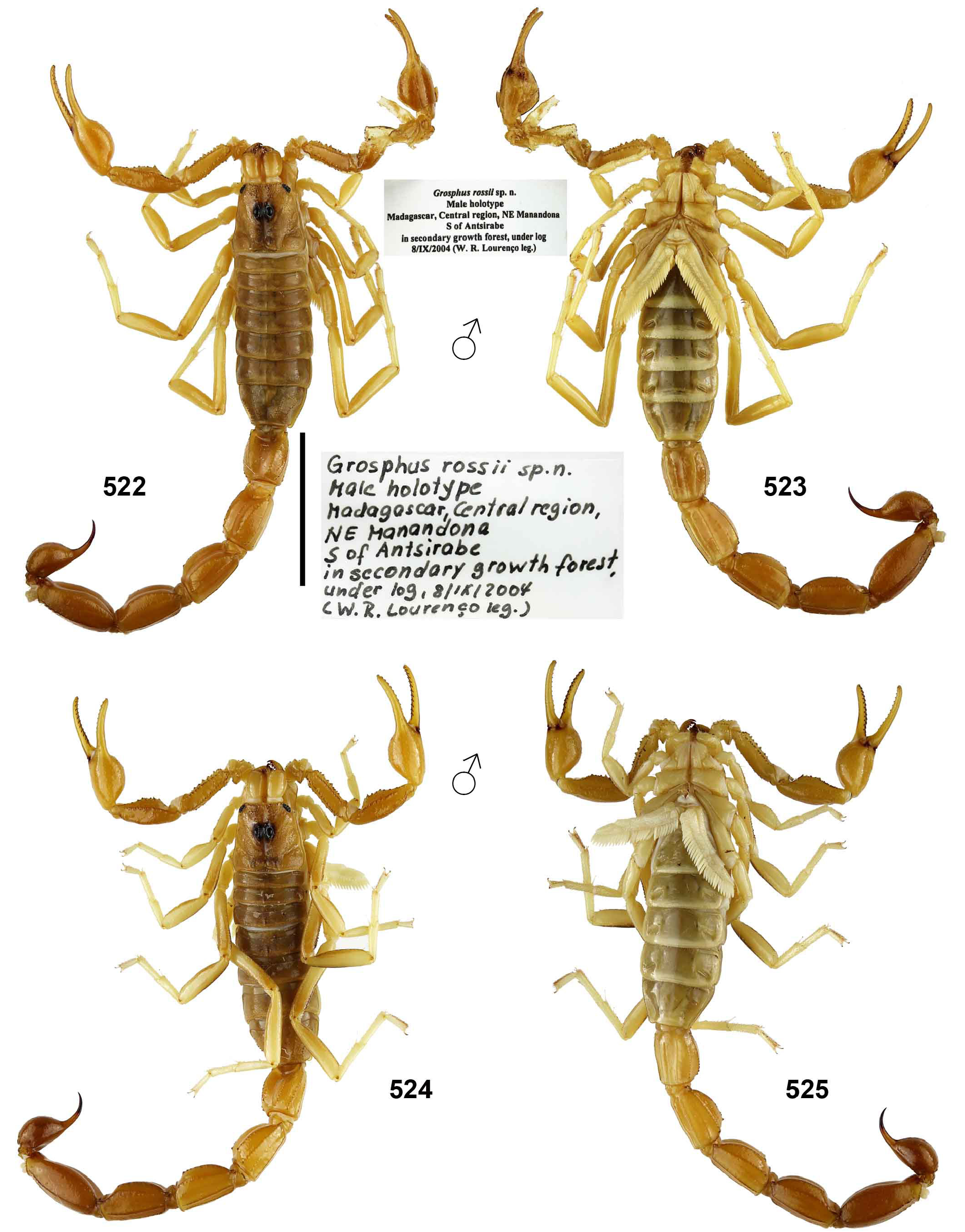
Grosphus rossii Lourenço, 2013 = Teruelius mahafaliensis ( Lourenço, Goodman & Ramilijaona, 2004) comb. n., syn. n.
Grosphus rossii was described by Lourenço (2013b) from a single adult male holotype collected from “ Central region, NE Manandona, S of Antsirabe, in secondary growth forest, under log, 8 August 2004, W. R Lourenço” and deposited in ZMUH. We loaned and studied the holotype and found that it was virtually identical in coloration, external morphological characters and morphometrics to Teruelius mahafaliensis ( Lourenço, Goodman & Ramilijaona, 2004) comb. n. Our comparative materials of the latter included a male collected near the type locality of that species ( Figs. 522–525 View Figures 522–525 ), and determined material loaned from FMNH including two adult males. In his description, Lourenço (2013b: 59) compared and contrasted G. rossii to T. limbatus , but not to T. mahafaliensis . A potential diagnostic difference is the pectinal tooth count (= 28) in G. rossii being lower than the range (35–40) reported for male T. mahafaliensis by Lourenço et al., (2007b: 373, tab. III). However, we examined a male T. mahafaliensis collected near the species type locality with PTC of 29–33 ( Figs. 524– 525 View Figures 522–525 ). The type locality of G. rossii on the central plateau at ca. 1400 m a. s. l., is in a cooler, more humid zone, quite far from the other records of T. mahafaliensis concentrated on the Mahafaly Plateau, a region of subarid thorn scrub along the southwest coast (ca. 120 m a.s.l.). It was suggested that G. rossii was evidence of microendemism. We take a more conservative position and interpret the very close morphologies of G. rossii and T. mahafaliensis as indicative of a eurytopic species with wider distribution. Broad elevation ranges of> 1400 m are known for some widely distributed scorpions that inhabit varied bioclimatic zones (e.g., Anuroctonus pococki Soleglad & Fet, 2004 , 300– 1850 m a.s.l., Soleglad & Fet, 2004; Bothriurus burmeisteri Kraepelin, 1894 and Brachistosternus weijenberghi (Thorell, 1876), 1000 –3000 m a.s.l., Campón et al., 2014; Compsobuthus maindroni (Kraepelin, 1901) , Hottentotta jayakari (Pocock, 1895) , Nebo omanensis Francke, 1980 and Orthochirus glabrifrons (Kraepelin, 1903) , 0–1850 m a.s.l., Lowe, 2010c). The status of G. rossii should be reviewed when topotypic females are collected and their basal pectinal tooth compared to that of T. mahafaliensis , as this is a more reliable diagnostic character in Teruelius gen. n.
Another potential synonym of T. mahafaliensis is T. magalieae ( Lourenço, 2014) . According to its description, the morphometrics, coloration, and meristics of T. magalieae are very close to those of T. mahafaliensis . The type locality of Cap Saint Marie ( holotype male as only known specimen) lies on the southwestern coast in the same bioclimatic region as the latter species. Lourenço (2014: 633) did not compare T. magalieae to T. mahafaliensis , but claimed that the most closely related species was G. rossii , which we here synonymize under T. mahafaliensis . The diagnostic differences between T. magalieae and G. rossii are not compelling: (i) pectines with 36 vs. 28 teeth (a range of variation allowed here for T. mahafaliensis ); (ii) pedipalp fingers with 12–13 vs. 12–12 granule rows (overlapping counts); and (iii) overall paler coloration (differences in color shade are not uncommon for different populations of a species inhabiting areas with different substrates). We provisionally list this species, until it can be critically evaluated by study of more material and analysis of variation. The female of T. magalieae is unknown, and the species might be better diagnosed if the female basal pectinal tooth were determined to be unique.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Teruelius
| Lowe, Graeme & Kovařík, František 2019 |
Grosphus
| LORIA, S. F. & L. PRENDINI 2018: 184 |
| LORIA, S. F. & L. PRENDINI 2014: 25 |
| PRENDINI, L. & L. ESPOSITO 2010: 675 |
| LOURENCO, W. R. & V. SOARIMALALA & S. M. GOODMAN 2007: 375 |
| VACHON 1940: 254 |