Pseudasthenes, Aleixo, Alexandre, Chesser, Terry, Jr, Remsen & Brumfield, Robb T., 2010
|
publication ID |
https://doi.org/10.5281/zenodo.294042 |
|
DOI |
https://doi.org/10.5281/zenodo.5678555 |
|
persistent identifier |
https://treatment.plazi.org/id/03BD382D-FFCC-911D-FF18-8867489EFB4B |
|
treatment provided by |
Plazi |
|
scientific name |
Pseudasthenes |
| status |
gen. nov. |
Pseudasthenes , genus nov.
Type species. Synallaxis patagonica d’Orbigny, 1839
Included species. Asthenes patagonica ( d’Orbigny 1839) , Asthenes cactorum Koepcke 1959 , Asthenes humicola ( Kittlitz 1830) , Asthenes steinbachi ( Hartert 1909) .
Diagnosis. We were unable to identify a synapomorphic phenotypic character for the genus, but the four species share the following features: small furnariids ( 15–22 g), with predominantly gray and brown plumage and no streaks on dorsal parts; gular patch feathers black and white, or dull orange ( P. cactorum ) but never a combination of black and orange; tail slightly longer than wing (tail/wing ratio 1.1–1.3), graduated (rectrix 6/ rectrix 1 ratio 0.55–0.70), and composed of 12 blunt rectrices with well-integrated barbs (except for the tip in some species). Phylogenetic diagnosis: the most inclusive crown clade that includes Asthenes patagonica and A. humicola but not Pseudoseisura lophotes or Spartonoica maluroides (d'Orbigny & Lafresnaye) (Baycapped Wren-Spinetail) .
Etymology. The generic name, from the Greek pseudo (false) and asthenes (insignificant, strengthless), denotes the outward resemblance of species of this genus to species of Asthenes but highlights the fact that they are not closely related. The name is feminine in gender.
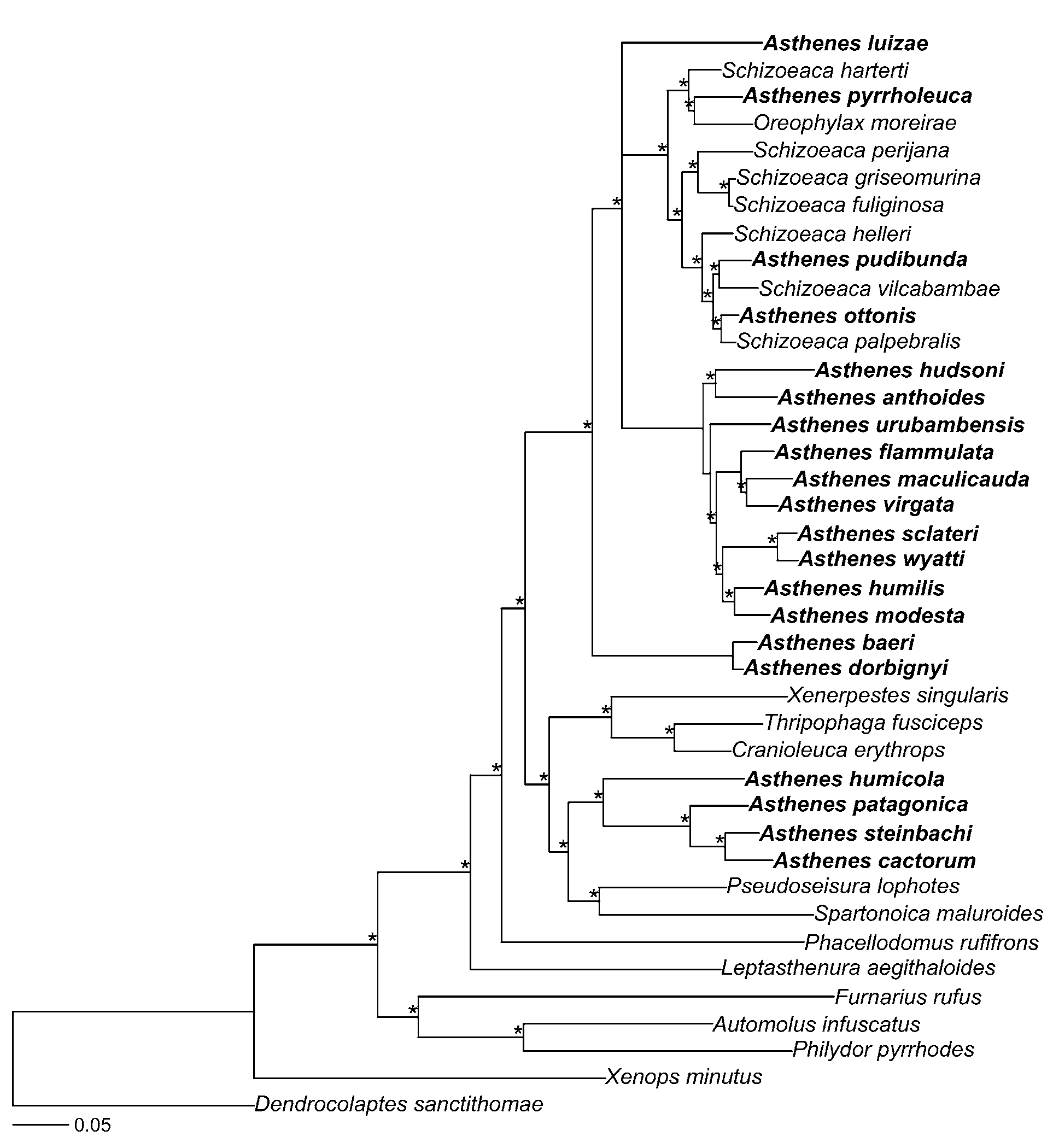
Genetic analyses. A preliminary genetic analysis of our data from all furnariid species found that Pseudasthenes was sister to a clade composed of Pseudoseisura and Spartonoica . This preliminary analysis also indicated that Asthenes , as currently recognized, is not monophyletic because Schizoeaca and Oreophylax are nested within it. To demonstrate that Asthenes is not monophyletic, and to propose a new hypothesis for phylogenetic relationships among Asthenes , Schizoeaca , and Oreophylax , we present an analysis of a subset of taxa from this larger study. This restricted analysis includes all species of Asthenes except A. heterura (Berlepsch) (Maquis Canastero) and A. berlepschi (Hellmayr) (Berlepsch’s Canastero), all species of Schizoeaca except S. coryi (Berlepsch) (Ochre-browed Thistletail), and Oreophylax moreirae (Ribeiro) (Itatiaia Spinetail) . We also included in the analysis the furnariids Furnarius rufus (Gmelin) (Rufous Hornero) , Leptasthenura aegithaloides (Kittlitz) (Plain-mantled Tit-Spinetail), Cranioleuca erythrops (Sclater) (Redfaced Spinetail) , Thripophaga fusciceps Sclater (Plain Softtail) , Phacellodomus rufifrons (Wied-Neuwied) (Rufous-fronted Thornbird), Spartonoica maluroides , Xenerpestes singularis (Taczanowski & Berlepsch) (Equatorial Graytail) , Pseudoseisura lophotes , Philydor pyrrhodes (Cabanis) (Cinnamon-rumped Foliagegleaner), Automolus infuscatus (Sclater) (Olive-backed Foliage-gleaner), and Xenops minutus (Sparrman) (Plain Xenops ) ( Table 1 View TABLE 1 ). We used the dendrocolaptid species Dendrocolaptes sanctithomae (Lafresnaye) (Northern Barred Woodcreeper) to root the tree.
Tissue collections: AMNH—American Museum of Natural History, New York City, USA; DZUFMG—Coleção Ornitológica do Departamento de Zoologia da Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil; FMNH—Field Museum of Natural History, Chicago, USA; ICN—Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá, Columbia; LSUMNS— Louisiana State University Museum of Natural Science, Baton Rouge, USA; UFMG—Laboratório de Biodiversidade e Evolução Molecular da Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil; USNM—National Museum of Natural History, Smithsonian Institution, Washington, D.C, USA.
Using the Qiagen DNeasy kit, genomic DNA was extracted from 25 mg of pectoral muscle following the manufacturer's protocol. We amplified and sequenced three mitochondrial genes (ND3, CO2, and ND2), as well as the autosomal nuclear gene beta-fibrinogen intron 7 (Bf7), following methods described in Chesser et al. (2007). For at least one individual per genus, two additional nuclear protein-coding genes (RAG1 and RAG2) were sequenced. Most of the RAG sequences from were taken from Moyle et al. (2009); samples of Xenerpestes singularis , Pseudoseisura lophotes , and Oreophylax moreirae were amplified and sequenced for this study, according to methods described in Moyle et al. (2009). Following alignment and the exclusion of unique inserts from Bf7, the six-gene concatenated dataset included 6,972 base pairs.
In model-based phylogenetic inference, there is a trade-off between modeling the evolutionary process as closely as possible and the risk of over parameterization ( Sullivan & Joyce 2005; McGuire et al. 2007). In preliminary analyses using the Akaike Information Criterion ( Sullivan & Joyce 2005) we identified the General Time Reversible model of nucleotide substitution with gamma distributed rate variation across sites (GTR + Γ) and a fully partitioned dataset (a different model for each position of each coding gene [15] and the nuclear intron) as the best model and partitioning regime. We then performed a Bayesian analysis as implemented in MRBAYES 3.1 ( Altekar et al. 2004; Huelsenbeck & Ronquist 2001; Ronquist & Huelsenbeck 2003) on CIPRES Portal v1.15 ( Miller et al. 2009). The Bayesian posterior probability density was estimated by Metropolis-coupled Markov chain Monte Carlo in two independent runs. Each run consisted of four incrementally heated chains, continued for 25 million generations, and was sampled every 2500 generations. All chains reached stationarity, the independent runs converged (split frequencies <0.01), and all parameters met benchmark ESS values (>200) as evaluated in Tracer v1.4.1 ( Drummond & Rambaut 2007). After discarding the first 5 million generations as burn-in, we computed a majority-rule consensus tree. Following methods in Chesser et al. (2009), we also performed a Maximum Likelihood (ML) analysis. The best tree from this analysis was identical to the majority-rule consensus tree; therefore, we do not include the ML results here.
All individuals of Pseudasthenes formed a strongly supported clade (posterior probability = 1.0) sister to the Pseudoseisura-Spartonoica clade ( Fig. 1 View FIGURE 1. A ). All other Asthenes species (hereafter Asthenes sensu stricto), together with Oreophylax moreirae and all sampled species of Schizoeaca , formed a monophyletic group. These results demonstrate that Pseudasthenes and Asthenes sensu stricto do not form a clade. Although several authors have suggested that Asthenes is not monophyletic ( Pacheco et al. 1996; Zyskowski & Prum 1999; Remsen 2003; Vasconcelos et al. 2008), the pattern of relationships that we found had not been predicted.
Asthenes View in CoL is usually subdivided into at least two informal subgroups based on differences in plumage pattern, habitat, and nest architecture: a group of plain-plumaged species that inhabit deserts and dry forests and make nests of sticks, and a group of streaked species that inhabit grassy areas and make nests of grasses ( Pacheco et al. 1996; Collias 1997; Remsen 2003). We recovered a strongly supported clade that corresponds to the streaked group of canasteros. Members of this lineage include A. humilis View in CoL , A. wyatti (Sclater & Salvin) View in CoL (Streak-backed Canastero), A. sclateri (Cabanis) View in CoL (Puno Canastero), A. anthoides (King) (Austral Canastero) View in CoL , A. hudsoni (Sclater) View in CoL (Hudson’s Canastero), A. urubambensis View in CoL , A. flammulata (Jardine) View in CoL (Many-striped Canastero), A. virgata (Sclater) View in CoL (Junin Canastero), and A. maculicauda (Berlepsch) View in CoL (Scribble-tailed Canastero). In addition, we found that A. modesta (Eyton) (Cordilleran Canastero) View in CoL , a plain-looking species that constructs stick nests, is part of this clade.
On the other hand, the plain-plumaged, stick-nesting canasteros, considered by many to be a natural group, consist of at least two major clades (in addition to A. humicola View in CoL , discussed above). Four belong to the newly described genus Pseudasthenes . Two other species, A. dorbignyi (Reichenbach) View in CoL (Rusty-vented Canastero) and A. baeri View in CoL , form a clade sister to the remaining species of Asthenes sensu stricto ( Fig. 1 View FIGURE 1. A ). There is a resemblance in morphology and habits between these two Asthenes View in CoL species and Pseudasthenes . Further, they show a pattern of geographic replacement similar to that found in a species complex or even a superspecies ( Remsen 2003). After careful examination of specimens of these species, mostly study skins but also some skeletons, we were unable to find diagnostic characters that unequivocally separate A. dorbignyi View in CoL and A. baeri View in CoL from all species of Pseudasthenes , especially because of plumage similarities of the former with P. c a c t o r u m and P. steinbachi View in CoL .
After transferring the corresponding species to Pseudasthenes , Asthenes remains paraphyletic because Oreophylax moreirae and all species of Schizoeaca are nested within it. We found a well-supported clade (posterior probability = 1.0) that includes all species of Schizoeaca and Oreophylax as well as three longtailed species of Asthenes : A. pudibunda (Sclater) (Canyon Canastero) , A. ottonis (Berlepsch) (Rusty-fronted Canastero), and A. pyrrholeuca . The close relationship of the Schizoeaca thistletails and the monotypic genus Oreophylax is not surprising given their similarities in morphology, nesting behavior, habitat, and voice ( Vaurie 1980; Remsen 2003; B. Whitney, pers. comm.), and a close relationship between Asthenes and Schizoeaca was previously suspected on the basis of similar throat patch configurations ( Remsen 2003). However, the polyphyly of Schizoeaca with respect to the three long-tailed Asthenes listed above is surprising given the phenotypic distinctness of Schizoeaca , especially in tail morphology. Moreover, Schizoeaca species were considered homogeneous to the point of being treated as a single species in the past ( Vaurie 1980).
The position of A. luizae Vielliard (Cipo Canastero) within Asthenes sensu stricto was unresolved; this species formed a trichotomy with the two main Asthenes clades. Pearman (1990) proposed, on the basis of voice and plumage, that its closest relative might be A. dorbignyi or A. patagonica . Vas c on c el o s et al. (2008), summarizing this and additional evidence, noted that all traits that it shared with other Asthenes were potentially plesiomorphic and concluded that its sister species could not be determined from the phenotypic data available. In fact, our data indicate this species is not closely related to any of the other Asthenes , including the species mentioned by Pearman (1990) and Vasconcelos et al. (2008), and forms a separate lineage within Asthenes sensu stricto.
The relationships of the three species not included in this study can be inferred tentatively from phenotypic characters. Asthenes berlepschi is almost certainly closely related to A. dorbignyi and may be a subspecies of A. dorbignyi ( Cory & Hellmayr 1925; Bond & Meyer de Schauensee 1942; Fjeldså & Krabbe 1990). The second missing species, A. heterura , has been considered closely related to ( Cory & Hellmayr 1925; Bond 1945), sister species to ( Vaurie 1980), or conspecific with (Meyer de Schauensee 1966) A. pudibunda . Pearman (2001), however, noted that A. heterura is sufficiently similar to A. pyrrholeuca in plumage that they can easily be confused in the field and even in the hand. Because A. pudibunda and A. pyrrholeuca are not sister species the specific placement of A. heterura is better regarded as uncertain, although it probably belongs to the long-tailed Asthenes / Schizoeaca / Oreophylax clade. Schizoeaca coryi is similar to other Schizoeaca thistletails in plumage, tail structure, and habitat ( Remsen 1981, 2003), and it presumably forms part of the same clade.
We recommend the following provisional classification of Asthenes and Pseudasthenes , based on our phylogeny and the rationale provided above for the missing species:
TABLE 1. Tissue samples used in the genetic analysis.
| Taxon | Museum | Sample ID | Locality |
|---|---|---|---|
| Furnarius rufus | AMNH | DOT10431 | ARGENTINA: prov. Neuquén; depto. Confluencia, Centenario. |
| Leptasthenura aegithaloides | AMNH | DOT10306 | ARGENTINA: prov. Neuquén; depto. Anelo, Sierra Auca Mahuida. |
| Schizoeaca perijana | ICN | AMC879 | COLOMBIA: depto. Cesar; Mun. Manaure, Sábana Rubia. |
| S. fuliginosa fuliginosa | LSUMNS | B30039 | ECUADOR: prov. Carchi; 8 km W Tufino. |
| S. fuliginosa plengei | LSUMNS | B8233 | PERU: depto. Pasco; Millpo, E Tambo de Vacas on Pozuzo- Chaglla trail. |
| S. vilcabambae | FMNH | 390681 | PERU: depto. Junin; Cordillera Vilcabamba, headwaters Rio Pomureni. |
| S. griseomurina | LSUMNS | B34804 | PERU: depto. Cajamarca; Cordillera del Condor; Picorana. |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.