Psilorhynchus sucatio (Hamilton)
|
publication ID |
https://doi.org/10.11646/zootaxa.3686.2.5 |
|
publication LSID |
lsid:zoobank.org:pub:B384D416-CF39-4FAB-B7CD-20C7E667E109 |
|
DOI |
https://doi.org/10.5281/zenodo.6158488 |
|
persistent identifier |
https://treatment.plazi.org/id/03BD6841-040F-FFD4-BD8B-FBA024DBFDD1 |
|
treatment provided by |
Plazi |
|
scientific name |
Psilorhynchus sucatio (Hamilton) |
| status |
|
Psilorhynchus sucatio (Hamilton) View in CoL
Figs. 2–9 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9
Cyprinus sucatio Hamilton, 1822:347 , 393 [rivers of Northern Bengal; no types known]. Psilorhynchus sucatio View in CoL var. damodarai David 1953:248 , fig. 2 [ Type locality: 12 mi. below the confluence of the Barakar with the Damodar R., India; 15 syntypes?].
Material examined: 462 specimens. Ganges: AMNH 43096, 5 (2 c&s), 13.7–17.6 mm SL; ANSP 148728, 5, 14.0– 18.8 mm SL; FMNH 94284, 5, 17.1–19.0 mm SL; USNM 231694, 5, 14.7–19.6 mm SL; Bangladesh: Dinajpur, Tangon River at Thakurgaon, 200 m upstream from bridge, 26°1'60.0"N 88°25'60.0"E. BMNH 1985.9.16.35–38, 4, 48.5–52.7 mm SL; Nepal: Chitwan National Park, Narayani River. CAS 50200, 23, 17.6–49.3 mm SL; Nepal: Chitwan Valley, including Khagari Khola, 72 km E of and slightly N of Hetauda and 18 km SSE of Narangar. CAS 50245, 2, 36.9, 42.0 mm SL; Nepal: Chitwan Valley, small streams rising in Churia Hills or ranges and flowing N into Rapti River. CAS 50289, 40 (8 c&s), 14.0– 52.8 mm SL; Nepal: Chitwan Valley, Reu River near confluence with Rapti River. CAS 50316, 7, 41.0– 57.1 mm SL; Nepal: Chitwan Valley, at Kasa Darbar or Dabar. CAS 50335, 5, 38.4–64.9 mm SL; Nepal: Chitwan Valley, Dudara River, tributary to Rapti River (from Churia Hills or Ranges). KU 28616, 40, 27.5–56.8 mm SL; Nepal: Sunsari, Bhantabari, Koshi River, seepage at Bhantabari, 26°31'30.0"N, 86°58'23.9"E. KU 29155, 5, 27.8–32.1 mm SL; Nepal: Jhapa, Bhadrapur, Mechi River at Bhadrapur, 26°32'17.9"N 88°6'6.1"E. KU 28589, 12, 30.4–65.5 mm SL; Nepal: Sunsari, Koshi River, seepage at Koshi Tappu Wildlife Refuge, 26°37'23.9"N 87°1'59.9"E. KU 29380, 16 (3 c&s), 29.7–40.8 mm SL; Nepal: Udayapur, Phattepur, Trijuga River, downstream from irrigation dam at Phattepur, 26°44'24.0"N 86°55'54.1"E. KU 29200, 14, 30.8–40.7 mm SL; Nepal: Morang, Belbari, Lohandra River at Belbari, 26°39'36.0"N 87°24'42.1"E. KU 40642, 48, 42.0–56.0 mm SL; Nepal: Narayani, Chitwan, Budirapti Kohla at Bachauli-3 Baghmara, 27°35'45.8"N 84°29'10.6"E. KU 40664, 34, 41.0– 63.8 mm SL: Nepal: Narayani, Chitwan, tributary of the Rapti River, Chitwan National Park, 27°34'20.7"N 84°29'47.0"E. OSUS 16679, 1, 39.5 mm SL; Nepal: Chitwan, Khoyeri Khola River, 10 km E of Narayangarh on Raj–Marg highway. OSUS 15957, 5, 45.1–54.5 mm SL; Nepal: Sunsari, Sapta Koshi River, fish market in Itahari. OSUS 16588, 1, 53.5 mm SL; Nepal: Nawalparasi, Narayani River, upstream from Tiger Tops Tented Camp. OSUS 15546, 1, 34.4 mm SL; Nepal: Chitwan, Buri Rapta Khola River at Saurcha. OSUS 16148, 1, 46.6 mm SL; Nepal: Nawalparasi, Marayoni River at Lainda Ghat. OSUS 15840, 2, 21.5, 27.0 mm SL; Nepal: Nawalparasi, Narayani River at Tribeni Ghat (above Tribeni Barrage). OSUS 15782, 2, 32.4, 36 mm SL; Nepal: Chitwan, Khageri Khola River, 12 km E of Narayangarh on Raj–Marg highway. UMMZ 205349, 42, 15.7–41.8 mm SL; Bangladesh: Dinajpur, Mahananda River at Tetulia, near location of Dak Bungalow, 26°28'60.0"N, 88°19'60.0"E. UMMZ 205352, 22, 11.1–50.7 mm SL: Bangladesh: Dinajpur, Keratoya River at Bhajanpur, just downstream from Indian border, 26°28'0.00"N 88°28'60.00"E. USNM 274804, 13, 28.4–48.7 mm SL; Nepal: Chitwan National Park, forest stream draining in to Rapti River. USNM 274788, 1, 53.9 mm SL; Nepal: Chitwan National Park, River running past Gaida Game Lodge, upstream of junction with Rapti River. Brahmaputra: AMNH 19648, 2 (1c&s), 28.3, 34.3 mm SL; India: West Bengal, Tista River at Sevoke. BMNH 1932.9.19.1–3, 3, 64.7–66.7 mm SL; CAS-SU 41719, 1, 67.1 mm SL; CAS-SU 28700, 1, 64.7 mm SL; India: West Bengal, Siliguri. BMNH 2010.2.2.1–4, 4 (2 c&s; 1 SEM), 25.0-30.0 mm SL; India: West Bengal, Jalpaiguri District, Jorai River at Laskarpara, Barobisha town outskirts, 26°28'52.3"N 89°49' 29.9E. NRM 52730, 2, 33.4– 33.5 mm SL; India: Assam, Kaloni, Rani Garden (Ranigodam; ornamental fish farm and tea garden), 26°3'42.0"N 91°37'54.0"E. UMMZ 205346, 47, 19.2–55.5 mm SL; Bangladesh; Rangpur, Ghaghat River, 6 km E of Rangpur on Badarganj road, 25°45'0.0"N 89°28'60.0"E. UMMZ 244850, 1, 40 mm SL: India: West Bengal, Tista River at Tista Barrage, 26°45'10.0"N 88°34'11.0"E. UMMZ 244899, 4, 44.0–59.0 mm SL; India, West Bengal, Korola River in vicinity of Jalpaiguri, 26°32'42.0"N 88°42'32.0"E. UMMZ 244921, 3, 50.0–54.0 mm SL; India: West Bengal, Panga River at Krishibagan, 26°28'22.0"N, 88°42'8.0"E. ZMH 3212, 2, 56.7, 59.8 mm SL; India: Assam, Raimona. Meghna: FMNH 93596, 4, 43.5–63.8 mm SL; UMMZ 205339, 20, 46.3–73.3 mm SL; Bangladesh: Rangapani Khal, creek, 6 km NNW of Jaintapur on Sylhet–Shillong highway, 25°10'0.0"N 92°5'60.0"E. Sangu: UMMZ 205338, 5, 20.2–22.5 mm SL; Bangladesh: Chittagong, Chittagong Hill Tracts, Sangu River and small tributary creek ca. 2 km upstream from Bandarban, 22°13'0.0"N 92°11'60.0"E.
Diagnosis. Psilorhynchus sucatio is distinguished from all other species of Psilorhynchus by the presence (vs. absence) of anteriorly directed radii over the anterior field of body scales, by its small pectoral fins (pectoral-fin length equal to pelvic-fin length vs. pectoral-fin length greater than pelvic-fin length), by having the rostral cap separate from the upper lip around the corner of the mouth (vs. rostral cap and upper lip continuous around corner of mouth), the posterior margin of the rostral cap triangular (vs. rounded), the shape of the second infraorbital (tube-like in lateral view, composed of sensory canal ossification only vs. plate like in lateral view), the shape of the basihyal (long, rod-like ossification vs. short spatula-like ossification), and by the absence (vs. presence) of a post-epiphyseal fontanelle. It is further distinguished from all species, except P. balitora , by having a complete covering of scales on its ventral surface between paired fins (vs. ventral surface between paired fins partially scaled, with a triangular to rectangular shaped scaleless patch along the ventral midline or with three to six pairs of flap-like structures supported by highly modified scales), and from all species except P. h o m a l o p t e r a by the absence (vs. presence) of the supraorbital bone. It is further distinguished from all members of the P. balitora and P. nudithoracicus species groups by its lower number of branched dorsal-fin rays (8 vs. 9–10), from all members of the P. nudithoracicus and P. homaloptera species groups by its lower number of principal caudal-fin rays (8–10+7– 9, most commonly 9+9, vs. 9–10+8–9, most commonly 10+9) and from all members of the P. homaloptera group by its lower number of lateral line scales (33–36 vs. 39–48) and unbranched pectoral-fin rays (4–5 vs. 7–11).
Description. General body shape as in Figures 3–7 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 . Morphometric data are listed in Table 1 and select meristic characters in Tables 2 and 3. Body elongate, dorsal profile arched, rising moderately to dorsal-fin origin, sloping gently towards caudal peduncle. Body depth greatest at dorsal-fin origin, narrowest at base of caudal peduncle. Ventral profile straight from lower jaw to anal-fin origin, weakly concave from anal-fin origin to caudal-fin base
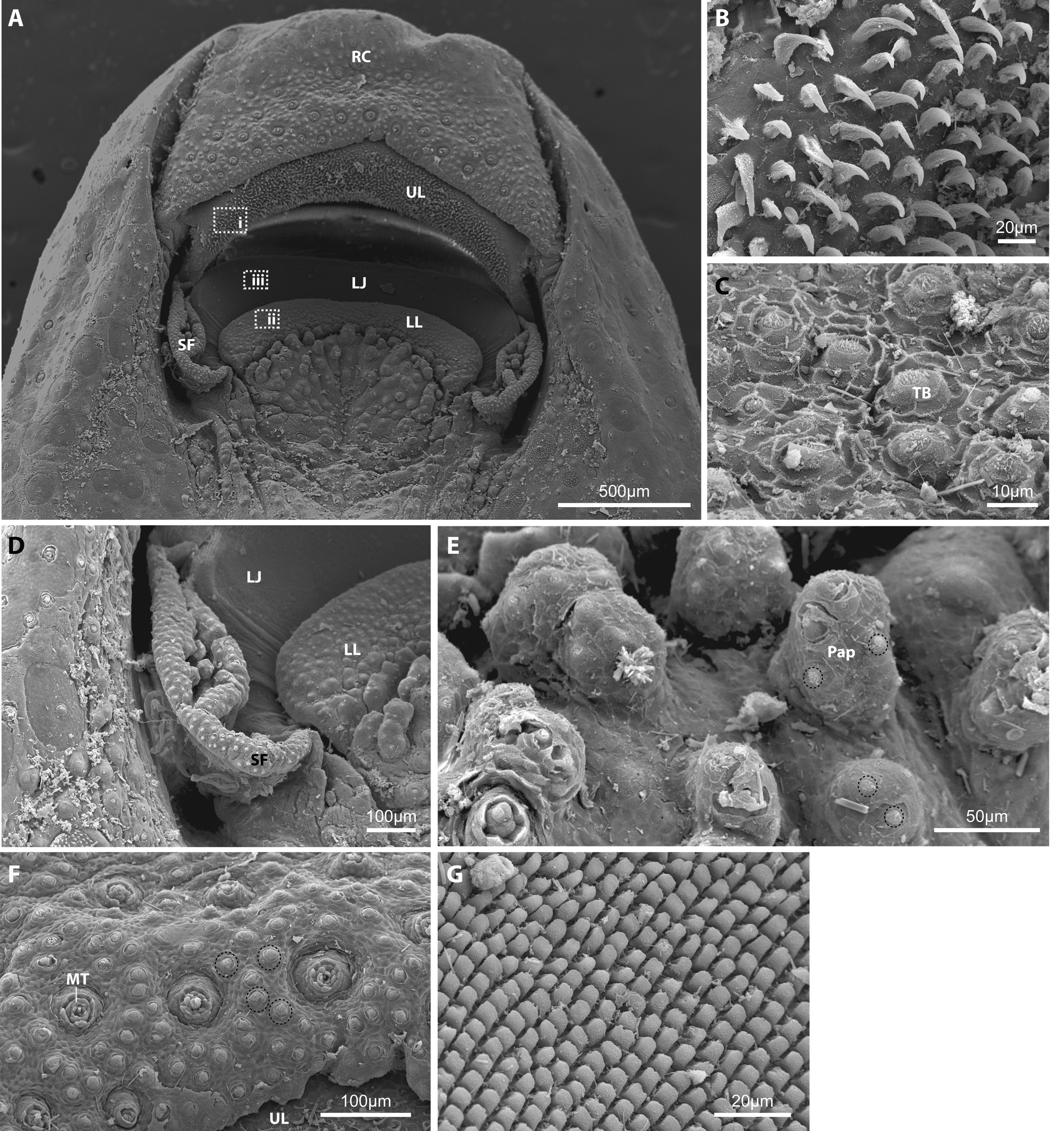
Head and eye large, pupil ovoid, narrowest ventrally ( Fig. 8 View FIGURE 8 ). Mouth inferior, snout long, roughly half of head length, rounded anteriorly, its ventral surface bordered by a deep longitudinal groove on each side. Rostral cap and upper lip fused, separated by a shallow groove ( Fig. 8–9 View FIGURE 8 View FIGURE 9 ); posterolateralmost part of rostral cap not continuous around corner of mouth, without contact to skin fold at posterolateral corner of mouth; posterior margin of rostral cap with obvious central indentation, giving anterior margin of upper lip a triangular appearance, with apex situated along ventral midline ( Fig. 8 View FIGURE 8 , 9 View FIGURE 9 A). Lower-jaw cushion, rounded anteriorly, with concave depression at center; superficial layer of lower-jaw cushion covered in globular papillae; globular papillae rimming posterior edge of lower lip larger than those situated more posteriorly. Upper lip covered with narrow, dagger-like unculi, ranging in height from ~10–20 μm; lower jaw covered in low, cube-like unculi, ~5 μm in height and width. Skin fold around posterolateral corner of mouth continuous anteriorly with posterolateralmost part of upper lip, not in contact with rostral cap; skin fold relatively smooth, non-papillated, without unculi or tubercles. Posterior edge of rostral cap, lower lip, skin fold, and globular papillae all densely covered with tastebuds ( Fig. 9 View FIGURE 9 ). Gill membranes joined to isthmus.
Pre-epiphyseal fontanelle small, triangular. Post-epiphyseal fontanelle absent. Five infraorbital bones (IO1-5). IO1 platelike, largest of the series. IO2–5 narrow tube-like bones, composed of sensory canal ossification only. Openings in preopercular portion of preopercular mandibular canal 5. Openings in nasal portion of supraorbital canal 3. Openings in parietal portion of temporal canal 2. Anguloarticular portion of preopercular mandibular canal, parietal portion of supraorbital canal and supraoccipital portion of temporal canal absent. Fifth ceratobranchial with a single row of four needle-like teeth. Hyoid bar with three branchiostegal rays; anteriormost greatly reduced in size. Basihyal a long, slender rod-shaped bone, extending far anterior of hyoid bar commissure along ventral midline. Anterior swimbladder chamber surrounded by a thick peritoneal tunic, partially enclosed in a bony capsule formed anteriorly by lateral process of 2nd vertebral centrum and laterally by outer arm of os suspensorium. Posterior swimbladder chamber greatly reduced in size.
Dorsal-fin rays ii.8 (48). Anal-fin rays iii.5 (3) or ii.6 (42). Principal caudal-fin rays 8+8 (1), 9+8 (12), 9+9 (29), 10+8 (1) or 10+9 (1), dorsal procurrent rays 3 (4), 4 (8) or 5 (2), ventral procurrent rays 3 (8), 4 (4) or 5 (1). Pectoral fin rays iv.7 (5), iv.8 (21), iv.9 (20) or v.8 (2), pelvic-fin rays ii.6 (1) or ii.7 (47). Paired fins horizontally oriented, roughly equal in size. Pectoral fin not reaching vertical through dorsal-fin origin, reaching three to five scale rows anterior to pelvic-fin origin when adpressed. Pelvic-fin origin posterior to dorsal-fin origin, insertion of anteriormost point opposite second branched dorsal-fin ray. Well-developed unculiferous paired-fin pads present along ventral surface of four anteriormost pectoral-fin rays and two anteriormost pelvic-fin rays. Dorsal fin high, triangular in shape with weakly pointed tip; posterior margin weakly concave. Anal fin relatively large, triangular in shape with weakly pointed tip; posterior margin weakly concave; not reaching caudal-fin base when adpressed. Caudal fin moderately forked, tips of upper and lower lobes weakly rounded.
Scales cycloid, large, with three or four well-developed radii over anterior and posterior fields of scale body. 33 (3), 34(15), 35 (22) or 36 (3) scales along lateral line, plus 1 (11), 2 (28) or 3 (5) on base of caudal fin. 3.5/1/2 transverse scale rows from dorsal-fin origin to pelvic-fin origin, 10 scale rows around caudal peduncle, 9 (2), 10 (9), 11 (31) or 12 (3) predorsal scales, 9 (5), 10 (28), 11 (14) or 12 (1) scales between anus and anal-fin origin. Ventral surface between paired fins scaled. Total number of vertebrae 34 (2), 35 (3), 36 (8), or 37 (2), consisting of 17+17 (1), 17+19 (1), 18+16 (1), 18+17 (3), 18+18 (7) or 18+19 (2) abdominal and caudal vertebrae.
Small conical tubercles with hard keratinized tip distributed over lateral and ventral surfaces of head, including snout and rostral cap, and along dorsal margin of orbit ( Fig. 8 View FIGURE 8 ). Tubercles present along posteroventral margin of rostral cap larger than those positioned more anteriorly on rostral cap and other surfaces of head. Head tuberculation more strongly developed in males. Scales situated posterior to occiput along dorsal midline and dorsolateral body surface edged with three or four small conical tubercles in males. Strip of minute conical tubercles present along dorsal surface of unbranched pectoral-fin rays in males. Single row of minute conical tubercles present along dorsal surface of unbranched and anteriormost branched pectoral-fin rays in females and anteriormost branched pectoral-fin ray in males. Anteriormost branched pectoral-fin ray also with single row of minute conical tubercles along ventral surface in both sexes. Dorsal surface of anteriormost pelvic-fin ray and lateral surface of last unbranched dorsal-fin ray with single row of minute conical tubercles in males only. Irregularly placed minute conical tubercles also present throughout paired-fin pad along ventral surface of anteriomost pelvic-fin ray of males.
Coloration. In alcohol body background color light cream ( Fig. 3–7 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 ). Occiput uniformly brown. Pigmentation features along dorsal surface posterior to occiput variable. Anterior to dorsal fin, majority of specimens with a faint brown blotch along dorsal midline, situated two to three scales posterior to occiput, and a small but prominent dark brown spot situated at anteriormost point of dorsal fin. Several specimens exhibit irregularly shaped brown markings (blotches or short streaks) between these two latter features, which may also be situated along the dorsal midline or situated slightly lateral to the midline. Below dorsal fin, majority of specimens exhibit a large brown saddle, extending along dorsal midline between last unbranched and 6th branched dorsal-fin rays. Posterior to dorsal fin, majority of specimens exhibit a single brown saddle (smaller in size than saddle situated below dorsal fin), situated along dorsal-midline just anterior to midway point between dorsal fin and caudal-fin base. Several specimens exhibit irregularly shaped brown markings anterior and posterior to post-dorsal saddle. Saddle situated below dorsal fin extending ventrad 1–2 scale rows on body side, separate from round to squarish brown blotches arranged in a longitudinal row on flank. Dorsal saddle situated between dorsal fin and caudal-fin base not extending on to lateral surface of body.
Flank with 6–8 (most commonly 7) round to squarish dark brown lateral blotches arranged in a longitudinal row. In specimens with 7, first situated posterodorsal to opercular opening, extending beneath lateral-line scales 2/ 3–6/7, second extending beneath lateral-line scales 9/10–12/13, third extending beneath lateral-line scales 17/18– 19/20, fourth extending beneath lateral-line scales 21/22–23/24, fifth extending beneath lateral line scales 25/26– 27/28, sixth extending beneath lateral line scales 29/30–32/33, seventh situated at base of caudal fin, extending beneath lateral-line scales 34/35–35/36. Scales in lateral line scale row and scale row directly below lateral line scale row with large chocolate brown melanophores concentrated at scale center, giving appearance of a horizontal stripe along lower half of flank. Stripe most prominent in juvenile specimens, occluding lower portion of round to squarish dark brown blotches arranged in a longitudinal row along flank. In larger specimens (over 30 mm SL) stripe is less prominent. Scales in rows dorsal to lateral line scale row with fine dusting of light brown melanophores at center. Scales in lowermost scale row on body (excluding scale situated at pelvic-fin origin) without pigmentation, providing a striking contrast to ventral edge of horizontal stripe along lower portion of flank. Unscaled base of pectoral fin and scale in lowermost body scale row situated at pelvic-fin origin peppered with small dark brown melanophores, forming indistinct pectoral-base and pre-pelvic spots, respectively. Small dark brown melanophores forming indistinct strip between tip of snout and anterior margin of orbit. Skin over opercle densely scattered with dark brown melanophores.
Table 3. Summary of pores in the cephalic sensory in select members of Psilorhynchus . See text for explanation of numbers. Absence of a particular canal is denoted by a “-”.
Otic Preopercular mandibular Infraorbital canal Supraorbital canal Canal Temporal Canal Anguloarticular Preopercle IO1 IO2 IO3 IO4 IO5 Nasal Frontal parietal Parietal supraoccipital Ventral surface largely devoid of pigment except for brown melanophores along anterior edge of rostral cap, ventrolateral border of snout, and ventral margin of infraorbital series. Paired fins marked proximally with a small brown blotch, formed by melanophores located along interradial membranes between bases of anteriormost rays. Dorsal surface of anteriormost rays of pectoral and pelvic fins marked with 1–4 small brown blotches, giving paired fins a mottled appearance anteriorly. Anterior edge of dorsal fin with three dark brown markings; one positioned at base of anteriormost rays, formed by brown melanophores located along interradial membranes between fin-ray bases; second located midway along length of last unbranched ray and first branched ray, formed by melanophores positioned along lateral edge of rays; third located distally, close to tip of last unbranched ray and first branched ray, formed by melanophores positioned along lateral edge of rays. Anal fin hyaline or with one or two small brown blotches formed by melanophores positioned along lateral surface of anteriormost rays. Caudal fin pigmentation highly variable. Majority of specimens with dark brown blotch over base of central caudal-fin rays and irregular shaped dark brown marking on upper and lower lobes. In several specimens markings along lower lobe and blotch at base of caudal fin are confluent.
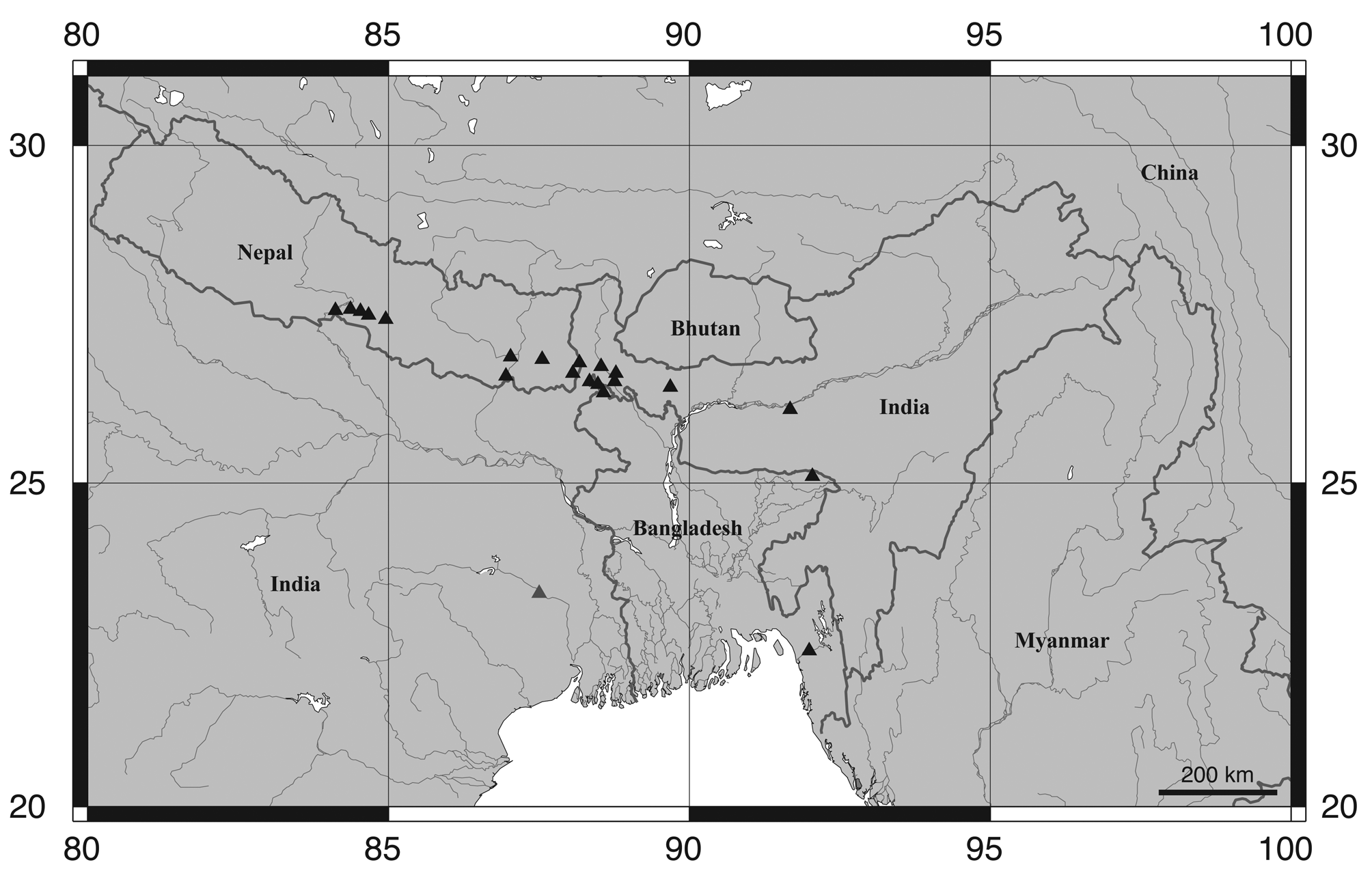
Distribution and habitat. Psilorhynchus sucatio is known with certainty from the Ganges River drainage in Bangladesh, India (West Bengal) and Nepal, the Brahmaputra River drainage in Bangladesh and India (Assam and West Bengal), and the Meghna and Sungu River drainages in Bangladesh ( Fig. 10 View FIGURE 10 ). David (1953) reported P. sucatio from the Damodar River drainage in India (southwestern West Bengal) and Arunachalam & Muralidharan (2008: 412) also report the presence of P. sucatio in the Indian states of Meghalaya and Tripura, without further comment. Rainboth (1983) reports that P. s u c a t i o is most commonly collected close to emergent or overhanging vegetation along the edge of sandy streams (based on collections made in Bangladesh).
Remarks. Psilorhynchus sucatio , as understood herein, is a widespread species, occurring throughout much of the Ganges and Brahmaputra drainages in northeastern India and northwestern Bangladesh, the Ganges drainage throughout southern Nepal ( Shrestha, 1980; Shrestha, 2008), and the Meghna and Sangu drainages in eastern Bangladesh ( Rainboth, 1983). Within India, Menon (1999) reports P. sucatio from the states of Assam, Meghalaya, Uttar Pradesh (possibly in reference to P. nudithoracicus ; see below) and Bihar, without mention of West Bengal. Arunachalam & Muralidharan (2008) also report P. s u c a t i o from Tripura and we have recently examined photographs of specimens collected from the Terei River (a tributary of Tlawng River; Barak drainage) in Mizoram. We have examined Indian material of P. s u c a t i o only from West Bengal (Ganges and Brahmaputra drainage) and Assam (Brahmaputra) and have not had the opportunity to examine material from Bihar, Meghalaya, Mizoram, or Tripura. The majority of the Bangladesh material of P. s u c a t i o that we have examined originates from the northwest of the country and is Gangetic or Brahmaputran in origin. We have had the opportunity to examine only relatively few specimens of P. s u c a t i o from the Meghna and Sangu drainages in eastern Bangladesh, with only juvenile specimens (< 25 mm SL) available from the latter (UMMZ 205338).
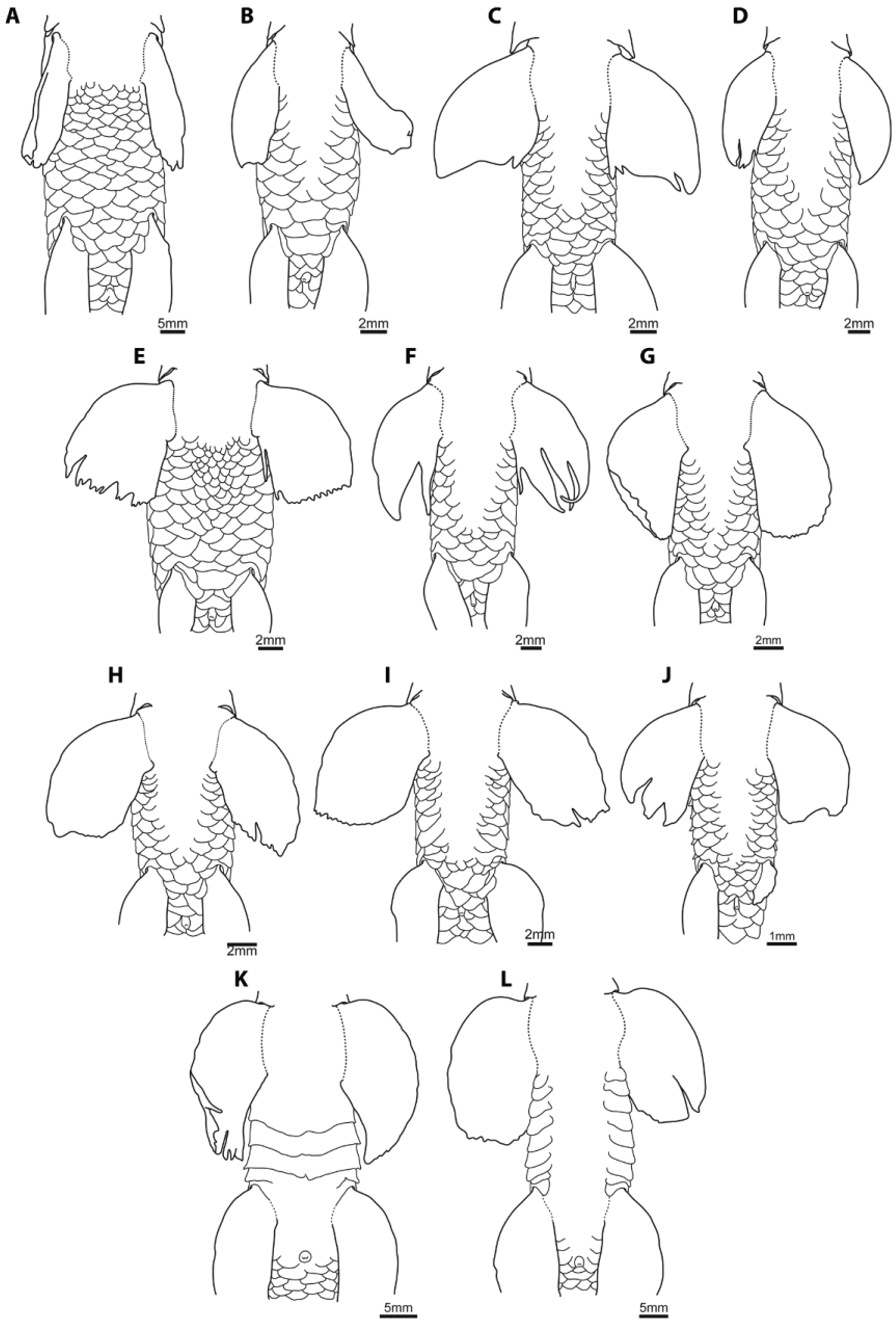
Throughout its range, P. s u c a t i o frequently occurs in sympatry with other species of Psilorhynchus , particularly members of the P. balitora and P. nudithoracicus species groups, including P. balitora , P. hamiltoni , P. nepalensis and P. nudithoracicus . Rainboth (1983) reports collecting P. s u c at io together with P. balitora and P. nudithoracicus at two Gangetic sites in northwestern Bangladesh, and with P. nudithoracicus at all other sites in Bangladesh (except one) at which the species was collected. At a locality on the Rapti River (Ganges River drainage) in central Nepal (Chitwan District), P. s u ca t io was collected together with P. n e p a l e n s i s and P. nudithoracicus (KWC, pers. obs), and with P. balitora and P. hamiltoni at a locality on the Tista River (Brahmaputra River drainage) in West Bengal (HHN, pers. obs). Even at small sizes, P. s u c a t i o can be distinguished easily from all members of the P. balitora and P. nudithoracicus species groups by its lower number of branched dorsal-fin rays (8 vs. 9–10), by the shape of the posterior margin of the rostral cap (which is triangular in P. sucatio vs. rounded in all congeners), and from all except P. balitora by the presence of scales along the ventral surface between paired fins (vs. ventral surface between paired fins partially scaled, with a triangular to rectangular shaped scaleless patch along the ventral midline; Fig. 12 View FIGURE 12 ).
The skeleton of P. s u c a t i o also differs markedly from other members of the genus (Conway, 2011), and several osteological features also serve as useful diagnostic features. The most easily assessed osteological character is the presence of anteriorly directed radii over the anterior field of body scales (Conway, 2011). In other members of the genus, anteriorly directed radii are invariably absent (Conway, 2011; Conway et al., 2012). The infraorbital series of P. sucatio also exhibits a number of autapomorphic features, including the shape of the second infraorbital, which is tube-like in lateral view and composed of sensory canal ossification only. In other members of the genus, the second infraorbital is plate-like, with well-developed bony lamellae dorsal and ventral to the sensory canal ossification (Conway, 2011). The basihyal of P. s u c a t i o is a long, rod-like ossification ( Ramaswami, 1952), which differs radically from the short, generally spatula-shaped (i.e., wider anteriorly than posteriorly) basihyal ossification present in other members of the genus ( Conway & Mayden, 2007; Conway, 2011). Lastly, P. sucatio is the only species of Psilorhynchus known to lack the post-epiphyseal fontanelle ( Fig. 13 View FIGURE 13 a). Though the size and shape of the post-epiphyseal fontanelle differs between and within the species groups of Psilorhynchus (particularly the P. balitora group; Fig. 13 View FIGURE 13 ), this structure is invariably present in all other members of the genus.
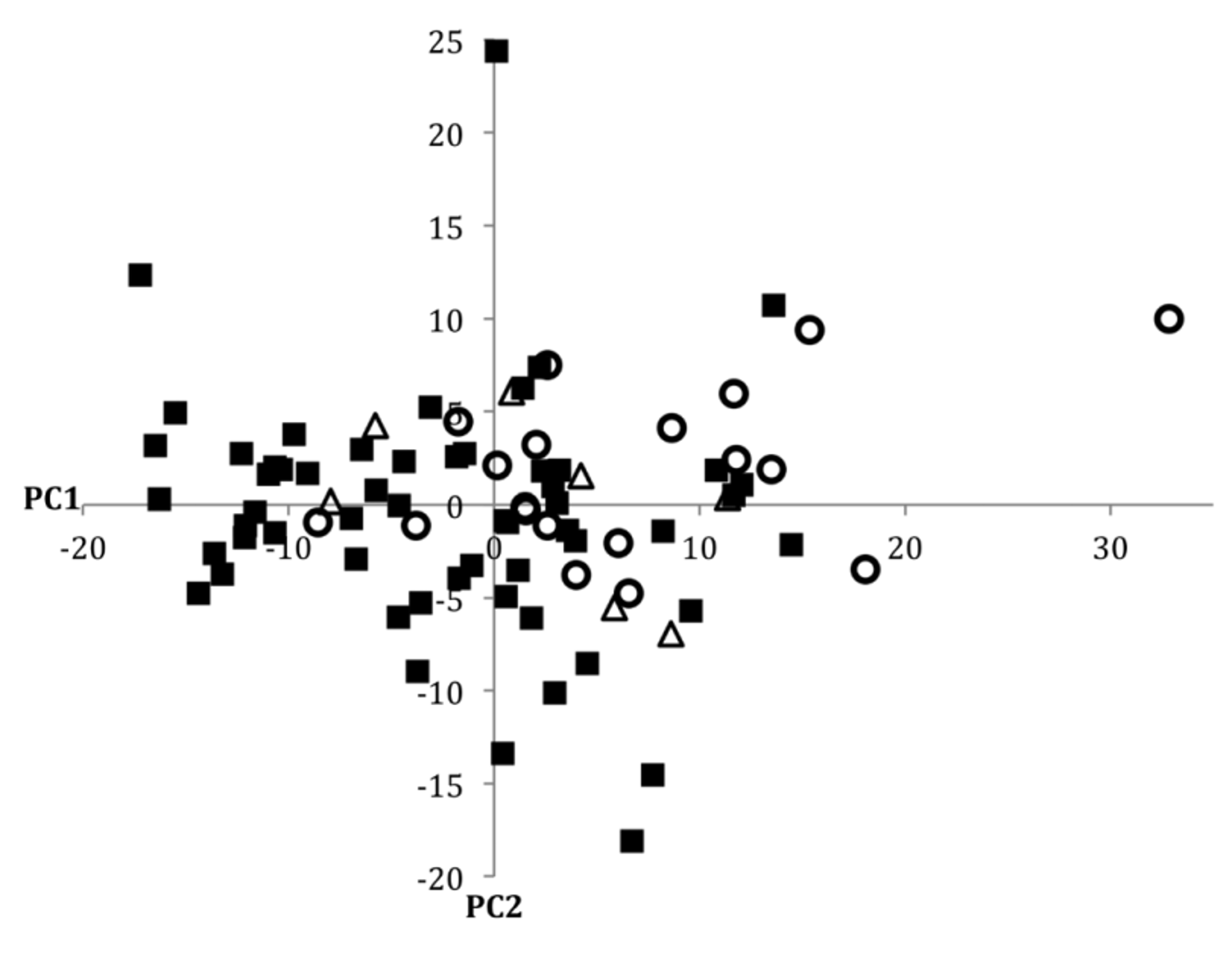
Despite its wide range, and somewhat variable coloration, we were unable to identify any consistent meristic differences between populations of P. sucatio from any of the four drainages for which we had the opportunity to examine material. In the size corrected principal components analysis of 21 morphometric characters for P. sucatio (including specimens from the Ganges, Brahmaputra, and Meghna drainages only), PC1 and PC2 accounted for 35% and 14% of the cumulative variation, respectively. All but one measurement (anus to anal-fin origin) exhibited positive loadings on PC1, with particularly strong loadings for caudal peduncle width (0.44), head depth (0.37) eye diameter (0.36), and mouth width (0.31). PC2 exhibited strong positive loadings for anal-fin length (0.41) and strong negative loadings for eye diameter (-0.53), caudal peduncle length (-0.40), and caudal peduncle width (- 0.32). In the scatterplot of PC1 vs. PC2 ( Fig. 14 View FIGURE 14 ) there is no obvious clustering of individuals by drainage, suggesting that there is little variation in the morphometric characters investigated between or within drainages.
Two subspecific taxa have been described for Psilorhynchus sucatio , namely P. s. damodarai, described as a “variety” of P. s u c a t i o from the Damodar River (West Bengal) by David (1953), and P. s. nudithoracicus , described as a subspecies from the tributary rivers of the Ganges throughout Uttar Pradesh by Tilak & Husain (1980). Each of these subspecific names has contributed to the synonymy of P. s. sucatio since Talwar & Jinghran (1991) and we follow this tradition here for P. s. damodarai (based solely on information contained in the original description) but question the current taxonomic status of P. s. nudithoracicus for several reasons. Firstly, striking differences exist between P. s. sucatio and P. s. nudithoracicus that are equivalent to those that exist between separate species of Psilorhynchus in the present classification (e.g., see Conway & Mayden, 2008a, b; Conway & Kottelat, 2007, 2010; Conway & Britz, 2010). Specifically, Tilak & Husain (1980) report that scales are present in P. s. nudithoracicus “all over the body except for the mid-ventral region, extending from the pectoral fins forwards up to the chin” ( Tilak & Husain, 1980: 37) and that the pelvic fins are shorter than the pectoral fins (“length of ventral [=pelvic fins] smaller but more than ¾ of the length of the pectoral”; Tilak & Husain, 1980: 39, table 1). In all specimens of P. s. sucatio that we have been able to examine, including material from the type locality (“Northern Bengal”), the ventral surface invariably has a complete covering of scales and the pectoral and pelvic fins are roughly equal in length ( Figs. 3–7 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 , 13 View FIGURE 13 A), which is in stark contrast to the condition present in congeners, in which the ventral surface between the paired fins is unscaled (except for P. balitora ; Conway & Mayden, 2008b; Fig. 13 View FIGURE 13 B–L) and the pelvic fins are shorter than the pectoral fins. Secondly, we are aware of no additional records of P. s u c a t i o from Uttar Pradesh other than the original report of P. s. nudithoracicus by Tilak & Husain (1980). The northwesternmost record for P. sucatio that we have been able to locate (and examine specimens from) is the Rapti River (Chitwan district) of central Nepal, leaving a clear separation between the range of P. s. sucatio and that of P. s. nudithoracicus in central Uttar Pradesh. Though we have not had the opportunity to examine material of P. s. nudithoracicus (all correspondences sent to the Zoological Survey of India by the first author to request permission to examine this material did not receive a response), given the clear morphological differences between P. s. nudithoracicus and P. s. sucatio documented in the original description of the former ( Tilak & Husain, 1980) and the non-overlapping distributional ranges between the two taxa, we conclude that the two are distinct from each other. We recommend that P. s. nudithoracicus be removed from the synonym of P. s. sucatio and elevated to full species status. A redescription of P. nudithoracicus is provided below, in which we develop the argument that this species is identical to P. gracilis and that P. nudithoracicus is the senior synonym and thus the valid name of this species.
| AMNH |
American Museum of Natural History |
| ANSP |
Academy of Natural Sciences of Philadelphia |
| FMNH |
Field Museum of Natural History |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| CAS |
California Academy of Sciences |
| OSUS |
Oklahoma State University |
| UMMZ |
University of Michigan, Museum of Zoology |
| CAS-SU |
California Academy of Sciences, Stanford University Collection |
| NRM |
Swedish Museum of Natural History - Zoological Collections |
| ZMH |
Zoologisches Museum Hamburg |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Psilorhynchus sucatio (Hamilton)
| Conway, Kevin W., Dittmer, Drew E., Jezisek, Laci E. & Ng, Heok Hee 2013 |
Cyprinus sucatio
| David 1953: 248 |
| Hamilton 1822: 347 |