Leydigia (Neoleydigia) acanthocercoides ( Fischer, 1854 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2082.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03BE87A4-4C7C-524C-CE97-E138795DF843 |
|
treatment provided by |
Felipe |
|
scientific name |
Leydigia (Neoleydigia) acanthocercoides ( Fischer, 1854 ) |
| status |
s. str. |
VIII. Leydigia (Neoleydigia) acanthocercoides ( Fischer, 1854) View in CoL s. str.
( Figs 224–261 View FIGURES 224–238 View FIGURES 239–249 View FIGURES 250–261 )
Lynceus acanthocercoides Fischer, 1854, p. 431 –433, Pl. 3: figs 21–25; Norman and Brady 1867, p. 34 –35.
Eurycercus acanthocercoides (Fischer) in Schodler 1862, p. 11.
Alona acanthocercoides (Fischer) in P.E. Müller 1867, p. 174, Pl. 4: fig. 5; Hellich 1877, p. 85; Daday 1884, p. 165; Daday 1885, p. 164.
Leydigia acanthocercoides (Fischer) View in CoL in Kurz 1875, p. 53 –54; Dybowski and Grochowski 1895, p. 150; Scourfield 1899, p. 171 –178, Pl. 11: figs 1–5; Lilljeborg 1901, p. 499 –502, Pl. 71: figs 4–8; Scourfield 1902, p. 30 –231, Pl. 11: figs 18–20; Daday 1903, p. 62; Keilhack 1909, p. 90; Spandl 1923, p. 29 –30, fig. 2; Bowkiewicz 1926, p. 59; Behning 1941, p. 290 –292, fig. 120; Herbst 1962, p. 90, fig. 72a–c; Šrámek-Hušek et al. 1962, p. 356 –358, fig. 133A–C; Manujlova 1964, p. 221 –222, Pl. 108: figs 1–4 (after Lilljeborg 1901); Scourfiled and Harding 1966, p. 38; Smirnov 1971, p. 458 –460, figs 569–570 (only Europe!); Flössner 1972, p. 327 –329, fig. 154; Prószyṅska 1978, p. 68; Sloka 1981, p. 88, fig. 67; Margaritora 1983, p. 129, figs 83A–C, G, 84A–B; Negrea 1983, p. 322 –324, fig. 132; Balvay 1984, p. 234; Hollwedel 1984, p. 69, fig. 5; Margaritora 1985, p. 271 –273, figs 15, 108; Dumont 1989, p. 139; Hollwedel 1995, Tab. 1; Røen 1995, p. 251–253, fig. 116; Flössner 2000, p. 355 –357, fig. 131A–G; Kotov et al. 2003b, p. 196, figs 86–90.
Alona balatonica Daday 1888, p. 95 –96, Pl. 1: figs. 51–52.
Chydorus clavatus Cosmovici, 1900, p. 158 –159, fig. 3.
Chydorus claratus Cosmovici, 1901, p. 305 noted in Smirnov (1971), but this publication is not cited in the reference list. Not seen by me.
Type locality. Fischer did not indicate the type locality accurately. Although his article was about Russian cladocerans, the material was from "stehenden Wässern der Insel Madeira, als auch in solchen bei Iwanofskoje in Gouvernment Tambow " ( Fischer 1854). Ivanovskoje in the Tambov Area, in the centre of European Russia , is a locality with no chance of being found; it is named after the most common Russian name, Ivan. Hundreds of localities with this name are found in every area of Russia.
Type material. Presumed lost.
Note on necessity of typification. The case of L. acanthocercoides is a good example of a difficult taxonomical situation, that must be partly resolved by the selection of a neotype (paragraph 75.3. of ICZN 2000). Perhaps populations from Madeira belong to L. ciliata – the most common African species. So it is necessary to select the neotype from a Russian locality. Howeber, in European Russian L. acanthocercoides is not so common as L. leydigi , and I had no good population from any locality close to Tambov, so selection of a neotype is a task for the future.
Material examined. Germany. Lake Dümmer, between Bremen and Osnabrück , Lower Saxony, coll. 28.07.1983 and 09.11.1983 by W. Hollwedel, tubes AAK 2001-031 and NNS 1998-253 ; Königswusterhausen bei Berlin, coll. by Hartwig, tube ZMHU 10755 View Materials . Sweden. Upsala, unknown collector, tube GOS F18517 . Hungary. Balaton, coll. by E. Daday, tube DAD D 1007-1894; II-489 ; Boglár, coll. by Vangel, tube DAD D 1212-1902; II-490 ; Csehi nagy tó, coll. by Vangel, tube DAD D 1212-1902; II-491 ; Czege, coll. in 1892 by E. Daday, tube DAD D 943-1892; II-492 ; Mezŏzáh, coll by E. Daday, tube DAD D 943-1892; II-670 (bad condition) . Kazakhstan. River Ayaguz , Semipalatinsk Area, coll. by T . S. Stuche , tube NMK 1049 View Materials . Russia ( European ). A pond in sport centre of AZLK factory near Metro Station Textilshchiky, City of Moscow, coll. 21.10.1998 and 19.09.2000 by A. Y. Sinev, tubes AAK 1998-065 and AAK 2003-017-18; Lake Krugloe , near Lotoshino , Moscow Area, coll. by N. M. Korovchinsky and M. A. Belyaeva (not saved) . Ukraine. Lake Burun, near Shchurovo, Krasnolimannij District , Donetsk Area, coll. 21.07.85 by O. Y. Lisatchev, tube NNS 1999-024 .
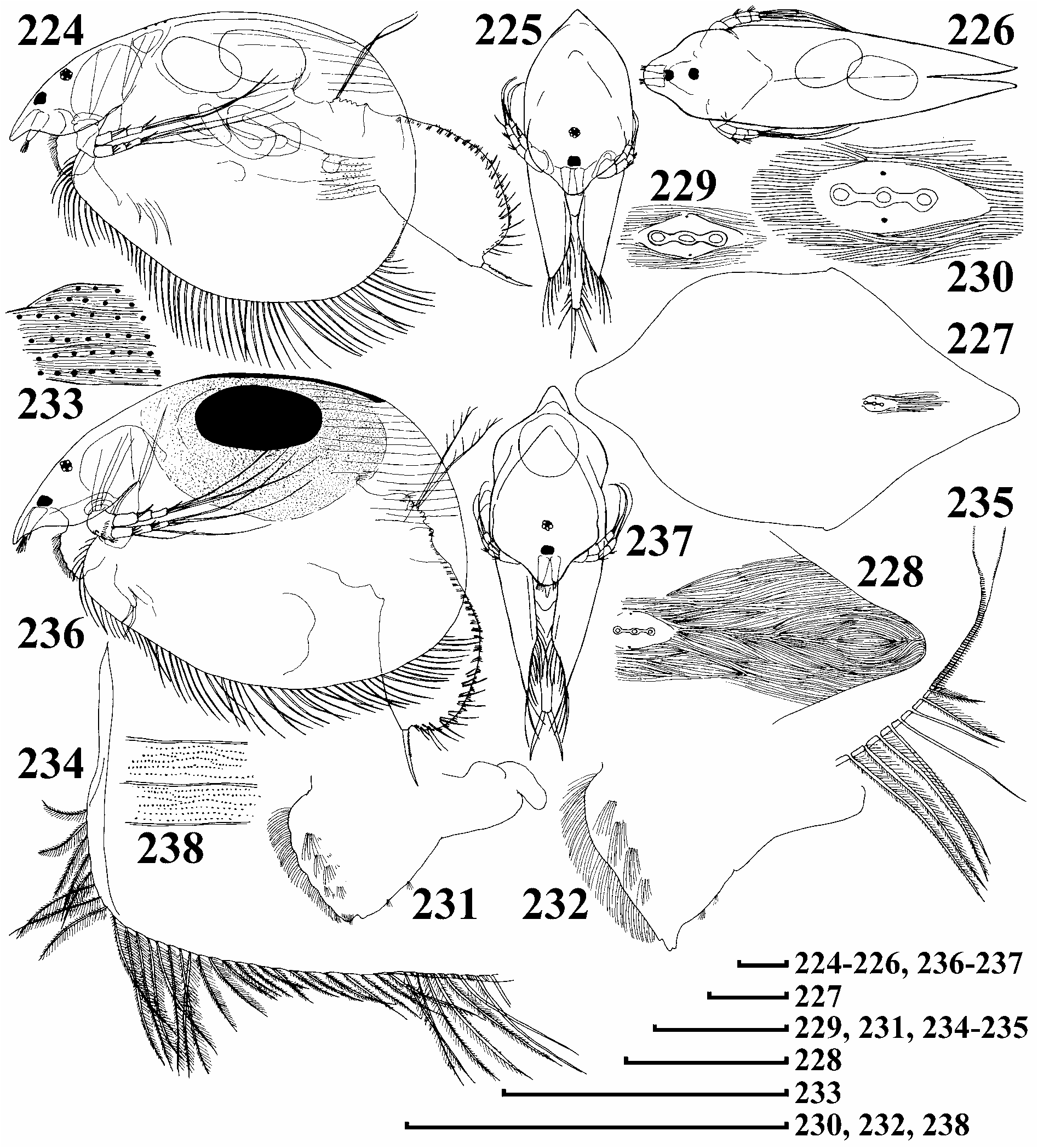
Diagnosis of European populations. Parthenogenetic female. Body subovoid to triangular-ovoid, maximal height in middle or posterior half, dorsal margin slightly and uniformly curved from tip of rostrum to completely smooth postero-dorsal angle; posterior margin convex ( Fig. 224 View FIGURES 224–238 ). Coarse striation on valves, clearly visible in adults and juveniles, fine striation on entire surface of valves and head shield very distinct ( Figs 228–230, 233 View FIGURES 224–238 ). In anterior view, body compressed laterally, dorsum like rounded triangle in section ( Fig. 225 View FIGURES 224–238 ), in dorsal view body subovoid, elongated ( Fig. 226 View FIGURES 224–238 ). Head small, compound eye small, ocellus slightly larger. Head shield with remarkably elongated posterior portion, PP = 8–10 IP, lateral head pores about 0.2–0.3 IP distance from midline, all head pores located on area devoid of reticulation ( Figs 227–230 View FIGURES 224–238 ). Labral keel widely-triangular-ovoid, with distinct apex, its posterior margin with two tufts of long setules, anterior margin with fringe of long setules from base to apex, also 4–5 lateral groups of fine, long setules, as long as marginal setules. On valves, setae at middle of ventral margin with sparser, shorter setulation, their bases located slightly submarginally, with long setules between them ( Fig. 234 View FIGURES 224–238 ). In posterior portion of the margin, setae setulated asymmetrically, posteriormost seta short ( Fig. 235 View FIGURES 224–238 ). Posterior to last marginal seta, a row of submarginal setules on inner face of posterior margin, in ventral part, these setules longer and in continuous series; in median and dorsal parts, located far from margin, and short, but larger than minute 'setules' of marginal membrane.
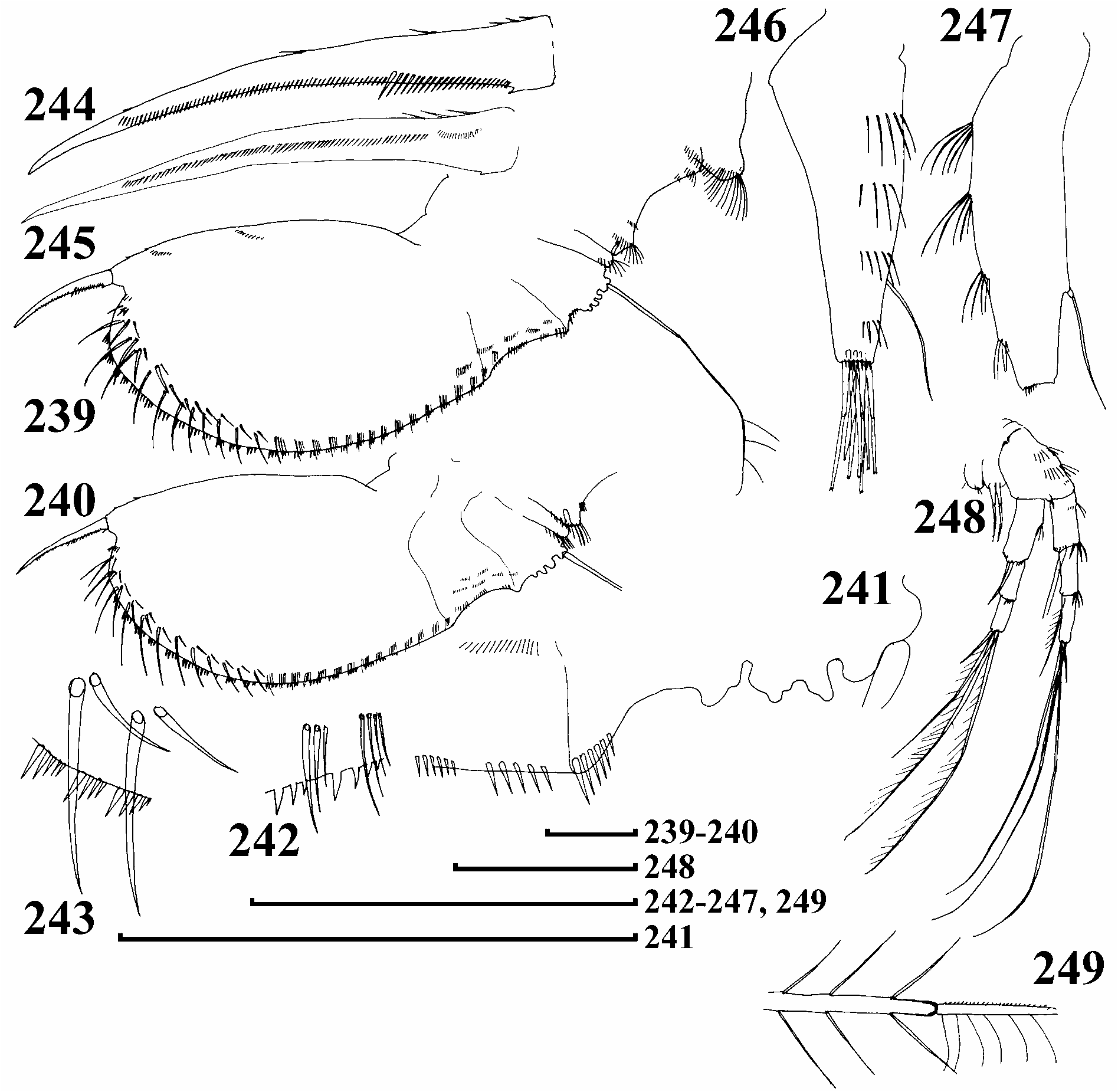
Postabdomen broad, subovoid, robust ( Figs 239–240 View FIGURES 239–249 ), preanal margin somewhat shorter than anus, with 3 relatively large projections in basal 2/3 ( Fig. 241 View FIGURES 239–249 ), preanal and postanal angles well defined, whole postanal margin from anus to basis of claws regularly arched, no distal margin and no dorso-distal angle. Postanal marginal denticles in numerous clusters, size increasing distally in each cluster ( Figs 242–243 View FIGURES 239–249 ), 11–14 fascicles of stout lateral setae, decreasing in size basally, normally 3–4 setae in each fascicle on distal portion, only 2 setae in each fascicle in middle, marginalmost seta significantly larger than adjacent one, 14–16 fascicles of lateral setules on basal half of postanal and anal margin. Postabdominal claw shorter than preanal plus anal portion of postabdomen, slightly curved, no setules at claw base, basal spine rudimentary, but normally present ( Figs 244 View FIGURES 239–249 ), setules in middle of inner face as other species ( Fig. 245 View FIGURES 239–249 ).
Antenna I elongate ( Figs 246–247 View FIGURES 239–249 ), not reaching tip of rostrum, with 4 transverse rows of long setules at anterior face and series of short setules at tip. Sensory seta arising about 1/4 of way from tip. Largest aesthetasc less than half length of appenfage, slightly projecting behind tip of rostrum. Antenna II with 3–4 stout spine-like setules on first and second endopod segments ( Fig. 248 View FIGURES 239–249 ). Apical swimming setae with basal segments bilaterally armed with fine, long setules, and asymmetrically armed distal segments, no chitinous insertions within distal segments ( Fig. 249 View FIGURES 239–249 ). Distal lateral seta short, basal lateral seta as long as apical seta.
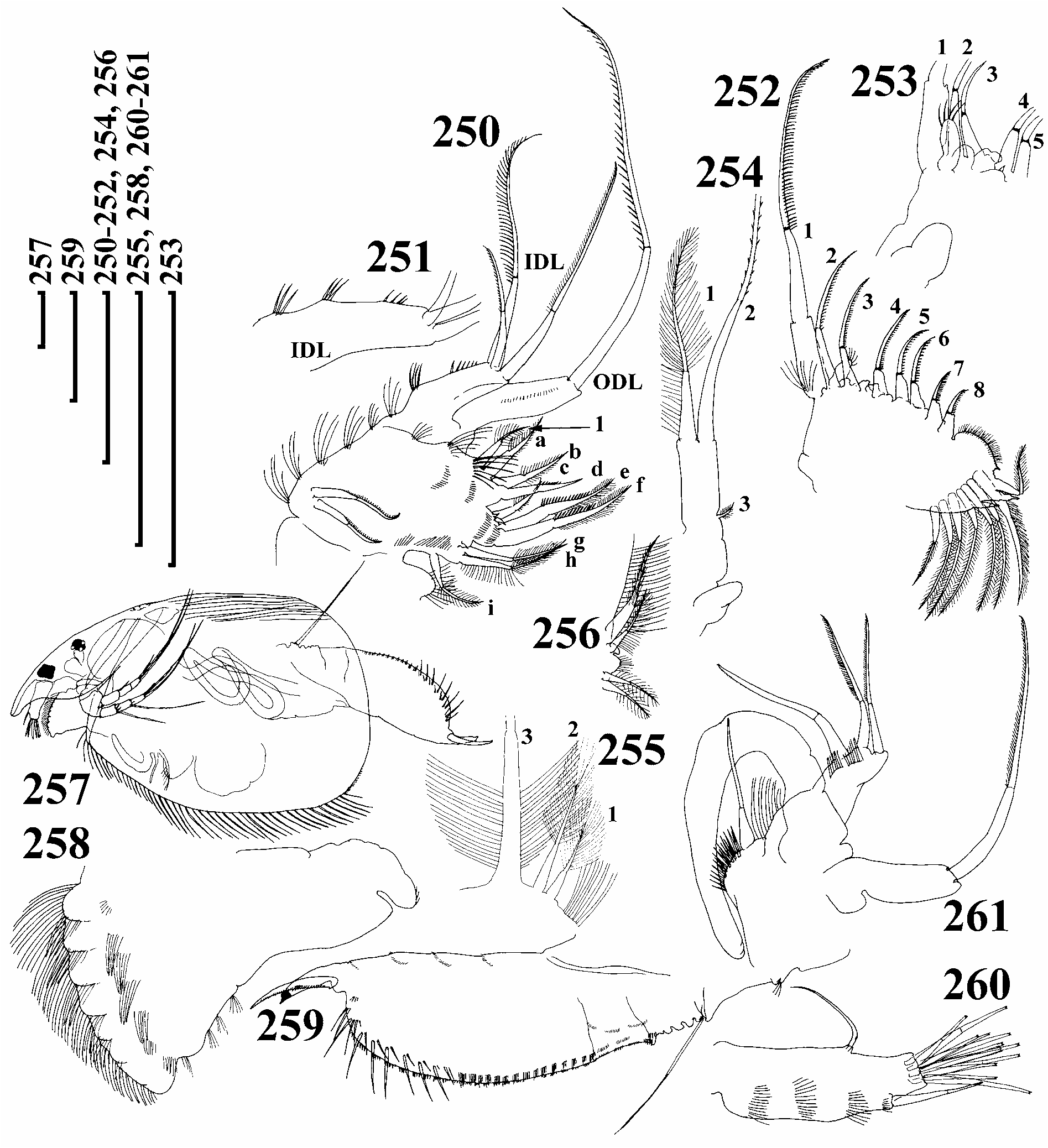
Trunk limb I with ODL large, elongate, conically narrowing distally, with row of fine setules and long seta with distal segment armed unilaterally with short, dense setules. IDL with 3 medial clusters of minute setules, and 3 marginal clusters of medium-sized setules ( Figs 250–251 View FIGURES 250–261 ), first seta smallest, with numerous short setules distally; second and third setae of unequal length, and differing in setulation, endite 3 with a medium-sized seta 1, armed with long, fine setules and small receptor near base, endite 2 setae e–f subequal, seta 2 rudimentary, naked, with sensillum near base, endite 1 with rudimentary seta 3. Two ejector hooks of subequal size. Trunk limb II with exopodite ovoid, small, supplied with a tuft of short, robust setules ( Figs 252–253 View FIGURES 250–261 ). Distalmost scraper 1 very long, with naked basal segment, on distal lobe with basal tuft of long setules. Limb III exopodite subquadrangular, with single, rudimentary lateral seta 3, and two distal setae (1–2) of unequal size, seta 2 with distal segment armed bilaterally with short setules ( Fig. 254 View FIGURES 250–261 ). Trunk limb IV exopodite with six setae, setae 2, and specially 1, short, but with long, fine setules ( Fig. 255 View FIGURES 250–261 ), in filter plate distalmost seta with inflated basal segment and fully setulated, all setae with inflated tips. On inner face of trunk limb V, distal seta longest, with naked basal segment and fully setulated distal segment, basal seta shorter, more slender, bi-laterally setulated distally and unilaterally armed basally, a hook near base, distal armature of gnathobase as a setulated lobe ( Fig. 256 View FIGURES 250–261 ).
Ephippial female. Shape basically similar to parthenogenetic female, dorsal wall of carapace additionally chitinised ( Fig. 236 View FIGURES 224–238 ); in anterior view, body with a thick dorsal keel, ( Fig. 237 View FIGURES 224–238 ), chamber for resting egg expands laterally. Ephippium relatively transparent, brown, with single resting egg. Entire surface of egg chamber chitinised, with sculpture of 'dots' ( Fig. 238 View FIGURES 224–238 ), which are in reality minute depressions on inflated wall of valve.
Adult male. My description is based on only two males, which were similar to those described by Lilljeborg (1901). Body low, subquadrangular, head larger than that in female, rostrum elongated, ocellus specially large ( Fig. 257 View FIGURES 250–261 ), labrum with numerous lateral groups of setules ( Fig. 258 View FIGURES 250–261 ). Postabdomen elongate, with ill-defined distal margin, postanal margin with small marginal denticles, about 9 groups of long lateral setae ( Fig. 259 View FIGURES 250–261 ). Penis thick, longer than half length of claw, distal end chitinised. Antenna I thick, with 4 rows of setules, sensory seta about 1/3 of way from distal end, thick male seta about 1/4–1/5 of way from distal end, 13 aesthetascs of varying length, largest half as long as appendage. Limb I with ODL bearing a single seta, shorter than in female and setulated with smaller, densely located setules. IDL with 2 setae as in female and a large male seta, smallest seta absent, copulatory hook long, U-shaped, seta of copulatory brush long, setules of copulatory brush robust.
Size. In my material, juvenile and adult parthenogenetic female 600–1090 µm (n = 10), ephippial female 920–1005 (n = 3) µm, adult male 615–645 µm (n = 2). According to Lilljeborg (1901), size of female up to 1100 µm, male up to 700 µm, Smirnov (1971) gave size of female up to 1000 µm.
Differential diagnosis. L. acanthocercoides is really a typical representative of this group, and has no unique traits as have L. laevis and L. ipojucae . In contrast to striata and ipojucae , the basal segment of the distalmost scraper of limb II is naked. European L. acanthocercoides differs from its closest relative L. ciliata in having an elongated posterior portion of the head shield, a broad postabdomen and only two lateral setae per lateral fascicle on the postabdomen.
Taxonomical comments. Apparently, there is only a single species of the acanthocercoides -group in Europe, and this has been well-studied previously. The description and illustrations of Lilljeborg (1901) were detailed, and have been reproduced many times by subsequent authors. By contrast, the status of Asian acanthocercoides -like populations is not yet clear. Authors from China illustrated acanthocercoides -like animals under different names (e.g. L. macrodonta louisi in Shen Chia-jui & Ta-hsiang (1962); L. ciliata in Shen Chia-jui et al. (1964); Leydigia acanthocercoides denticulatus Chen and Wang in Chen Shou-zhong et al. (1992)) with a broad postabdomen, but with 3–4 setae in each lateral bunch, among which the 2 marginalmost setae are long. This is perhaps a separate subspecies of acanthocercoides , or a separate species. The status of Chinese populations requires further consideration.
' Leydigia acanthocercoides ' described by Alonso (1996) is not member of the acanthocercoides -group, but a separate species (see above).
Alona balatonica Daday, 1888 was forgotten by its author. Daday (1903, p. 62) in a subsequent publication on the fauna of Balaton (type locality of A. balatonica ), mentioned only L. acanthocercoides and quadrangularis , and said nothing aboutn balatonica . Daday (1888) did not mention a basal spine on the postabdominal claw, so, it seems he had only L. acanthocercoides when he described balatonica .
Distribution. At present I can confirm the presence of this species only in Europe and northern Asian Russia ('Siberia').
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Genus |
Leydigia (Neoleydigia) acanthocercoides ( Fischer, 1854 )
| Kotov, Alexey A. 2009 |
Chydorus clavatus
| Cosmovici, L. C. 1900: 158 |
Alona balatonica
| Daday, E. 1888: 95 |
Leydigia acanthocercoides (Fischer)
| Kotov, A. A. & Van Damme, K. & Elias-Gutierrez, M. 2003: 196 |
| Flossner, D. 2000: 355 |
| Dumont, H. J. 1989: 139 |
| Margaritora, F. G. 1985: 271 |
| Balvay, G. 1984: 234 |
| Hollwedel, W. 1984: 69 |
| Margaritora, F. G. 1983: 129 |
| Negrea, S. 1983: 322 |
| Sloka, N. 1981: 88 |
| Flossner, D. 1972: 327 |
| Smirnov, N. N. 1971: 458 |
| Manujlova, E. F. 1964: 221 |
| Herbst, H. V. 1962: 90 |
| Sramek-Husek, R. & Strascraba, M. & Brtek, J. 1962: 356 |
| Behning, A. L. 1941: 290 |
| Bowkiewicz, J. 1926: 59 |
| Spandl, H. 1923: 29 |
| Keilhack, L. 1909: 90 |
| Daday, E. 1903: 62 |
| Scourfield, D. J. 1902: 30 |
| Lilljeborg, W. 1901: 499 |
| Scourfield, D. J. 1899: 171 |
| Dybowski, B. & Grochowski, M. 1895: 150 |
| Kurz, W. 1875: 53 |
Alona acanthocercoides (Fischer)
| Daday, E. 1885: 164 |
| Daday, E. 1884: 165 |
| Hellich, B. 1877: 85 |
| Muller, P. E. 1867: 174 |
Eurycercus acanthocercoides (Fischer)
| Schodler, J. E. 1862: 11 |
Lynceus acanthocercoides
| Norman, A. M. & Brady, G. S. 1867: 34 |
| Fischer, S. 1854: 431 |