Monatractides urania, Pešić, Vladimir & Smit, Harry, 2014
|
publication ID |
https://doi.org/10.11646/zootaxa.3820.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:BBE4177B-5A2C-4911-987C-454BB8FA767C |
|
DOI |
https://doi.org/10.5281/zenodo.6143481 |
|
persistent identifier |
https://treatment.plazi.org/id/03BFDC60-AB68-695C-FF47-FC26FBE5FF1C |
|
treatment provided by |
Plazi |
|
scientific name |
Monatractides urania |
| status |
sp. nov. |
Monatractides urania n. sp.
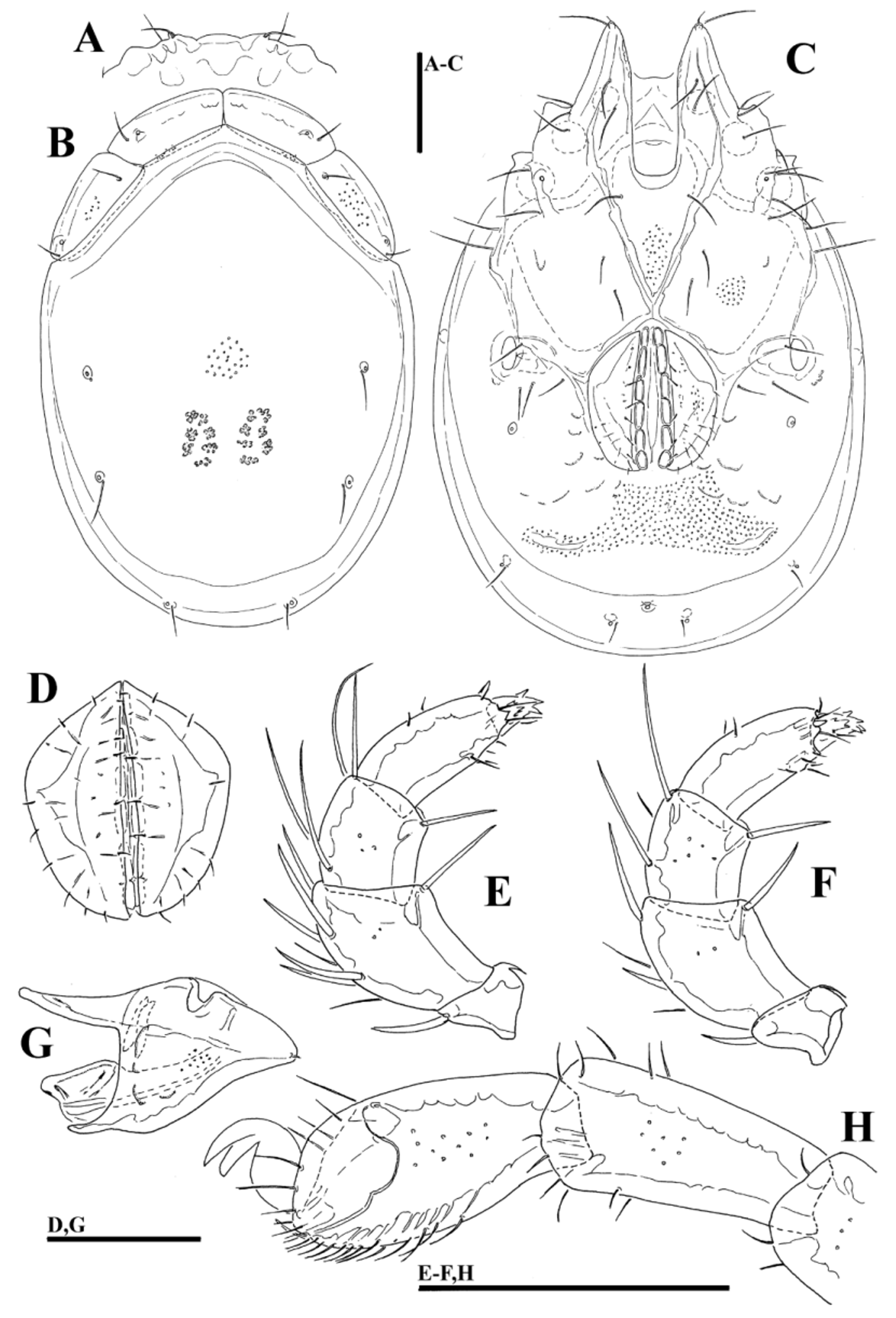
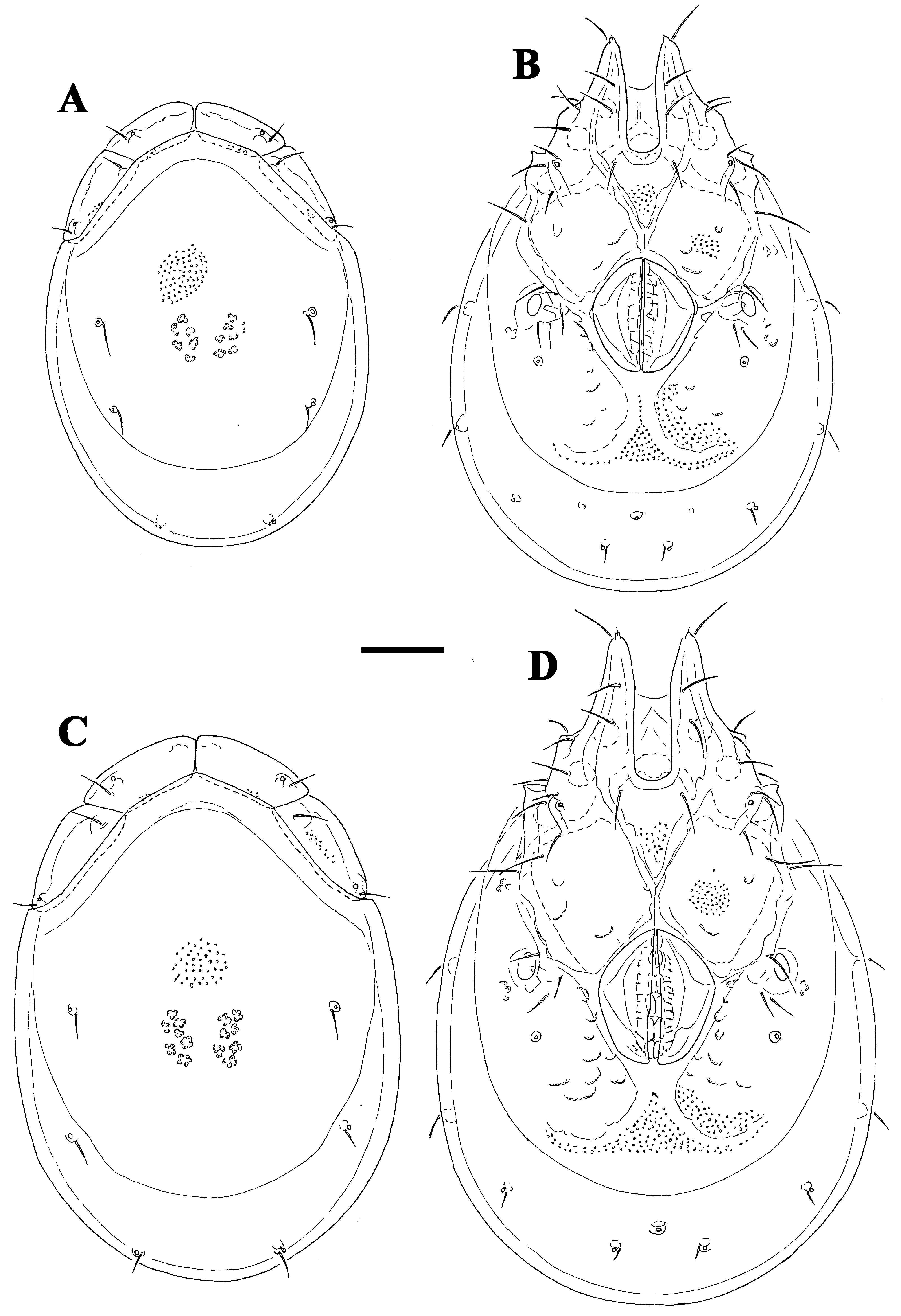
( Figs. 36A–H View FIGURE 36 A – H , 37I View FIGURE 37 A – I , 38I View FIGURE 38 A – I )
Type series. Holotype male, dissected and slide mounted, Ghana, Kintampo Falls, 8º 05.413 N, 1º 41.881 W, 3.iii.2011, Smit. Paratype: 3/0/0, same data as holotype, 2/0/0 dissected and slide mounted.
Diagnosis (Female unknown). Shoulder platelets shorter than frontal platelets (shoulder/frontal platelets L ratio 0.93–0.96); medial margin with convex protrusions medial to the eyes; medial margin of Cx-II/III very short (21µm); genital field large (L 140–160 µm) and strongly trapezoid in shape, anteriorly enlarged and pointed; I-L-6 L/H ratio 2.0–2.1, distally thickened.
Description. Male. General features —Idiosoma elongated-oval; shoulder platelets short, slightly shorter than frontal platelets (shoulder/frontal platelets L ratio 0.93–0.96); frontal margin medially convexly rounded between anterolaterally orientated apodemes ( Fig. 36A View FIGURE 36 A – H ); Cxgl–4 located far anteriorly, near tips of Cx-I; gnathosomal bay, U-shaped, proximally rectangular box-shaped; medial margin of Cx-II/III relatively very short; genital field large and strongly trapezoid in shape, anteriorly enlarged and pointed, laterally convex, tapering towards posterior ( Figs. 36C–D View FIGURE 36 A – H ); suture line of Cx-IV extending back beyond posterior margin of genital field; excretory pore slightly away from the line of primary sclerotization, excretory pore anterior to Vgl–2; gnathosoma nearly triangular in lateral view ( Fig. 36G View FIGURE 36 A – H ); P-2 and P-3 distal margins without pointed tips, ventral seta on P-4 short ( Figs. 36E–F View FIGURE 36 A – H ); I- L-6 L/H ratio 2.0–2.1, basally narrowed, distally thickened ( Fig. 36H View FIGURE 36 A – H ).
Measurements. Male ( holotype, in parentheses measurements of paratypes, n = 1)—Idiosoma (ventral view: Figs. 36C View FIGURE 36 A – H , 38I View FIGURE 38 A – I ) L 619 (642), W 425 (434); dorsal shield ( Figs. 36B View FIGURE 36 A – H , 37I View FIGURE 37 A – I ) L 525 (550), W 366 (384), L/W ratio 1.43 (1.43); dorsal plate L 491 (509); shoulder platelets L 116–119 (127–131), W 50–52 (55–58), L/W ratio 2.2–2.4 (2.3); frontal platelets L 123–125 (127–131), W 48 (50–52), L/W ratio 2.5–2.6 (2.5–2.6); shoulder/frontal platelets L ratio 0.93–0.96 (0.96). Gnathosomal bay L 150 (153), Cx-I total L 275 (306), Cx-I mL 125 (122), Cx-II+III mL 21 (21); ratio Cx-I L/Cx-II+III mL 12.9 (14.6); Cx-I mL/Cx-II+III mL 5.9 (5.8). Genital field L/W 141 (155)/127 (130), ratio 1.11 (1.19); distance genital field-excretory pore 133 (131), genital field-caudal idiosoma margin 181 (189). Gnathosoma vL 157 (155); chelicera total L 189 (198); palp total L 188–190 (188–191), dL/H, dL/H ratio: P-1, 26/20, 1.3 (26/21, 1.26); P-2, 57/38–39, 1.48 (54–55/37, 1.48); P-3, 38–39/32, 1.2 (38–39/31, 1.25); P-4, 49/ 22, 2.2 (52/21–22, 2.4); P-5, 18–19/11–12, 1.6 (18–19/12, 1.5); P-2/P-4 ratio 1.16 (1.04); dL of I-L-2–6: 63 (65), 76 (80), 97 (99), 92 (97), 100 (106); I-L-6 H 48 (52), dL/H I-L-6 ratio 2.08 (2.03).
Female: unknown.
Etymology. The species is named after Urania (Ancient Greek: Οὐρανία), one of nine Muses from Greek mythology, who was a patron of tragedy. The species name is a noun in apposition (in the nominative case).
Discussion. The combination of very short medial margin of Cx-II/III, the box-shaped gnathosomal bay, the large and strongly trapezoid genital field, makes the new species close to Monatractides ( M.) jucundus (Lundblad, 1927) (syn. M. afer (Lundblad, 1927)) , known from East Africa ( Lundblad 1952, Walter & Bader 1952). The male of latter species differs from M. urania n. sp., in its larger dimensions of idiosoma and palps, a frontal margin medially straight, a shoulder platelets longer than medial ones and a genital field anteriorly less pointed (see Lundblad 1952, figs. 38A–B).
Remarks. The ejaculatory complex of the male was not found, but sclerotized framework immediately above the genital field can be visible. For a discussion on the ejaculatory complex in M. jucundus see the following species.
Habitat. A sandy/bouldary streams, shaded by riparian vegetation.
Distribution. Ghana, known only from the type locality.
Monatractides microstoma ( Koenike, 1898) —species complex
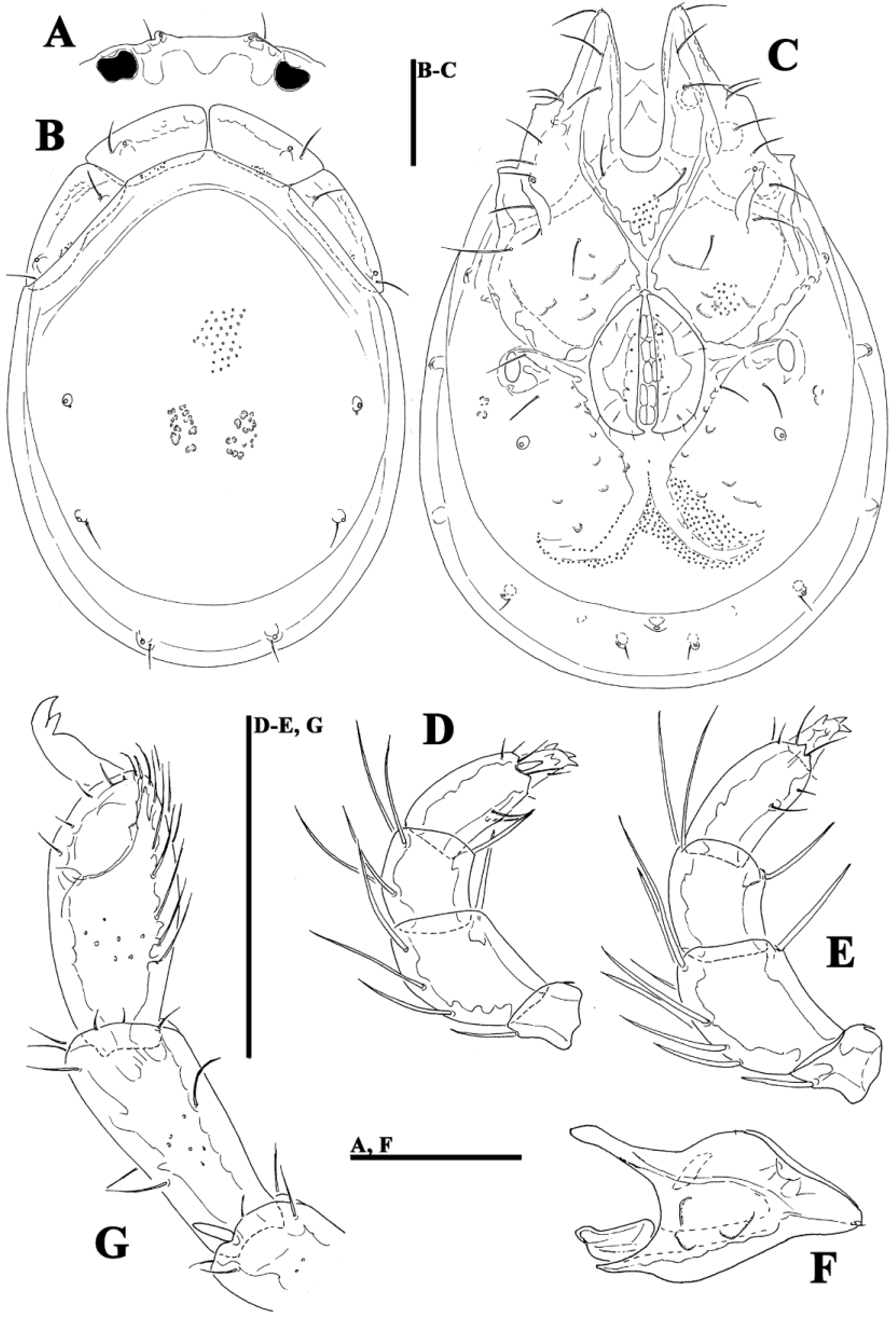
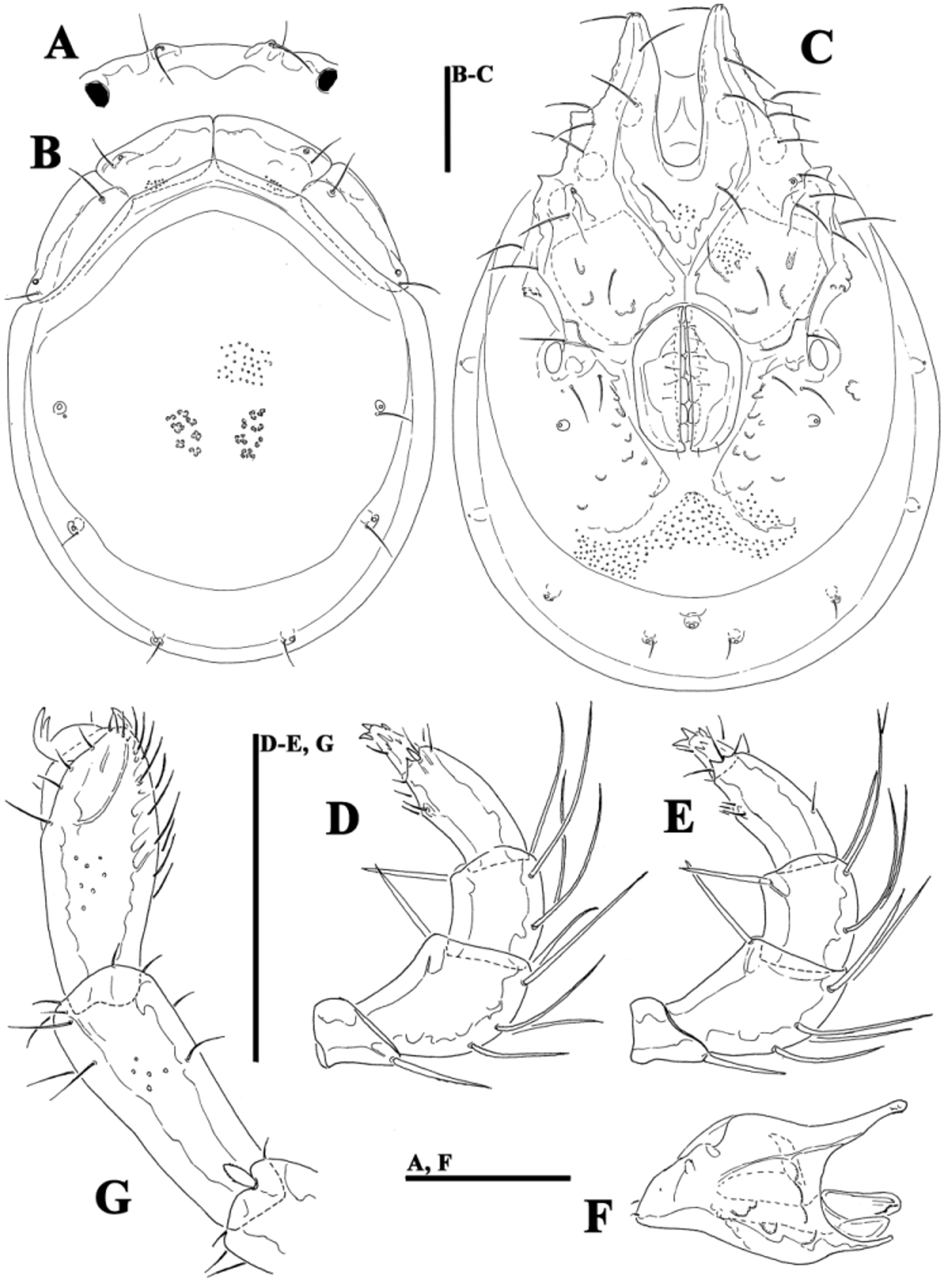
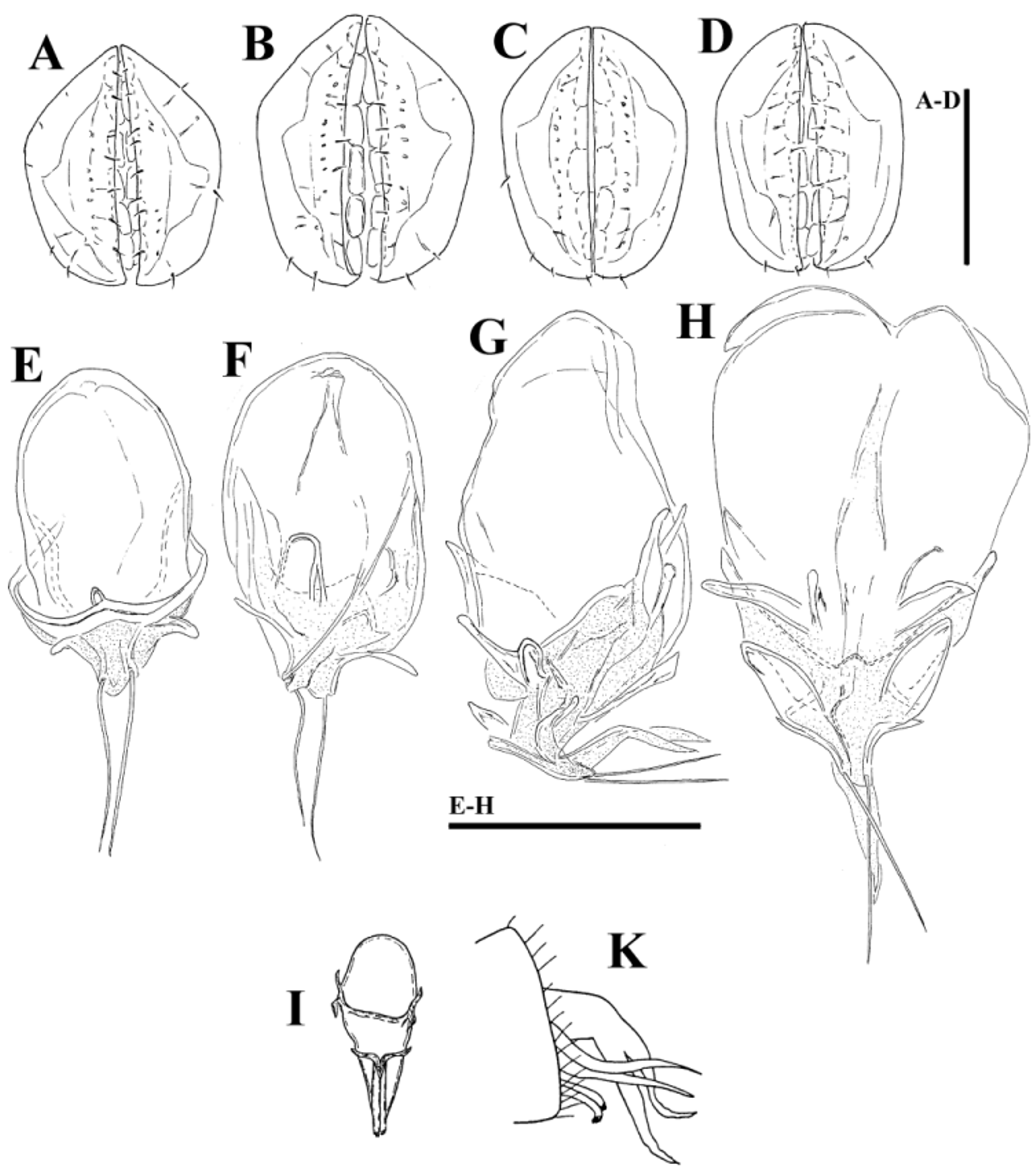
( Figs. 39A–G View FIGURE 39 A – G , 40A–G View FIGURE 40 A – G , 41A–D View FIGURE 41 A – D , 42A–H View FIGURE 42 A – K. A – H , 43A–I View FIGURE 43 A – I , 44E–L View FIGURE 44 A – L , 46A–C View FIGURE 46 A – C , Tabs. 4–5 View TABLE 4 View TABLE 5 )
New records. Ghana: Apkonu stream, downstream of falls, 6º 53.054 N, 0º 28.024 E, alt. 362 m asl., 21.ii.2013, Smit, 10/14/0 (2/2/0 Form ''A'' [2/1/0 mounted] + 8/13/0 Form ''B'' [6/2/0 mounted]); Small rainforest stream upstream Sagyimase, Atewa Hills, 6º 13.964 N, 0º 33.116 W, alt. 654 m asl., 8.iii.2011, Smit, 0/1/1 (Form ''B''); Kuluga River, N of Biakpa, 6º 51.365 N, 0º 25.101 E, alt. 388 m asl., 19.ii.2013, Smit, 0/1/0 (mounted) (Form ''B''); Kuluga River, upstream, hyporheic, 6º 51.223 N, 0º 25.141 E, alt. 410 m asl., 20.ii.2013, Smit, 4/3 (one juvenile)/0 (3/0/0 mounted) (1/0/0 Form ''A'' [mounted] + 2/3/0 Form ''B'' [2/0/0 mounted]); Agumatsa River at first bridge, Agumatsa Wildlife Sanctuary, 7º 06.830 N, 0º 35.760 E, alt. 253 m asl., 22.ii.2013, Smit, 5/9/0 (Form ''A''); stream Boti Falls, 6º 11.508 N, 0º 13.010 W, alt. 273 m asl, 9.iii.2011, Smit, 0/1/0 (Form ''B''); Kintampo Falls, 8º 05.413 N, 1º 41.881 W, alt. 235 m asl, 3.iii.2011, Smit, 8/7 (one juvenile)/0 (3/1/0 mounted) (4/5/0 Form ''A'' [1/1/0 mounted] + 4/2/0 Form ''B'' [2/0/0 mounted]); Afiaso stream, Kakum NP, 5º 30.087 N, 1º 26.373 W, alt. 114 m asl., 12.ii.2013, Smit, 0/1/0 (Form A); Fuller Falls, 8º 04.975 N, 1º 47.842 W, alt. 189 m asl, 6.iii.2011, Smit, 0/1/0 (Form ''A''); Supon stream near Asiakwa, Atewa Hills, 6º 15.530 N, 0º 30.642 W, alt. 250 m asl, 7.iii.2011, Smit, 1/0/0 (Form ''A''); Tagbo River, downstream of falls, 7º 00.708 N, 0º 34.326 E, alt. 394 m asl., 23.ii.2013, Smit, 19/11(one juvenile)/0 (6/2/0 mounted) (10/5/0 Form ''A'' [4/1/0 mounted] +8/6/0 Form ''B'' [2/1/0 mounted]); Akaa Falls, 6º 10.516 N, 0º 11.723 E, alt. 180 m asl., 9.iii.2011, Smit, 8/12/1 (1/0/0 mounted) (Form ''B''); Nubui River, Agumatsa Wildlife Sanctuary, 7º 06.986 N, 0º 35.548 E, alt. 254 m asl., 22.ii.2013, Smit, 10/14( 2 juveniles)/0 (Form ''A''); Laboun River, downstream of falls, Kyabobo NP, 8º 19.836 N, 0º 35.487 E, alt. 342 m asl., 24.ii.2013, Smit, 2/0/0 (1/0/0 Form ''A'' + 1/0/0 Form ''B''); stream downstream of falls, Wanjakli River, Likpe Todome, 7º 09.976 N, 0º 36.456 E, alt. 478 m asl., 23.ii.2013, Smit, 3/9/2 (1/1/0 Form ''A'' + 0/1/0 Form ''B'' mounted); Plunge pool Agumatsa Falls, Agumatsa Wildlife Sanctuary, 7º 06.350 N, 0º 36.476 E, alt. 258 m asl., 22.ii.2013, Smit, 12/8/0 (1/ 2/0 mounted) (Form ''A'').
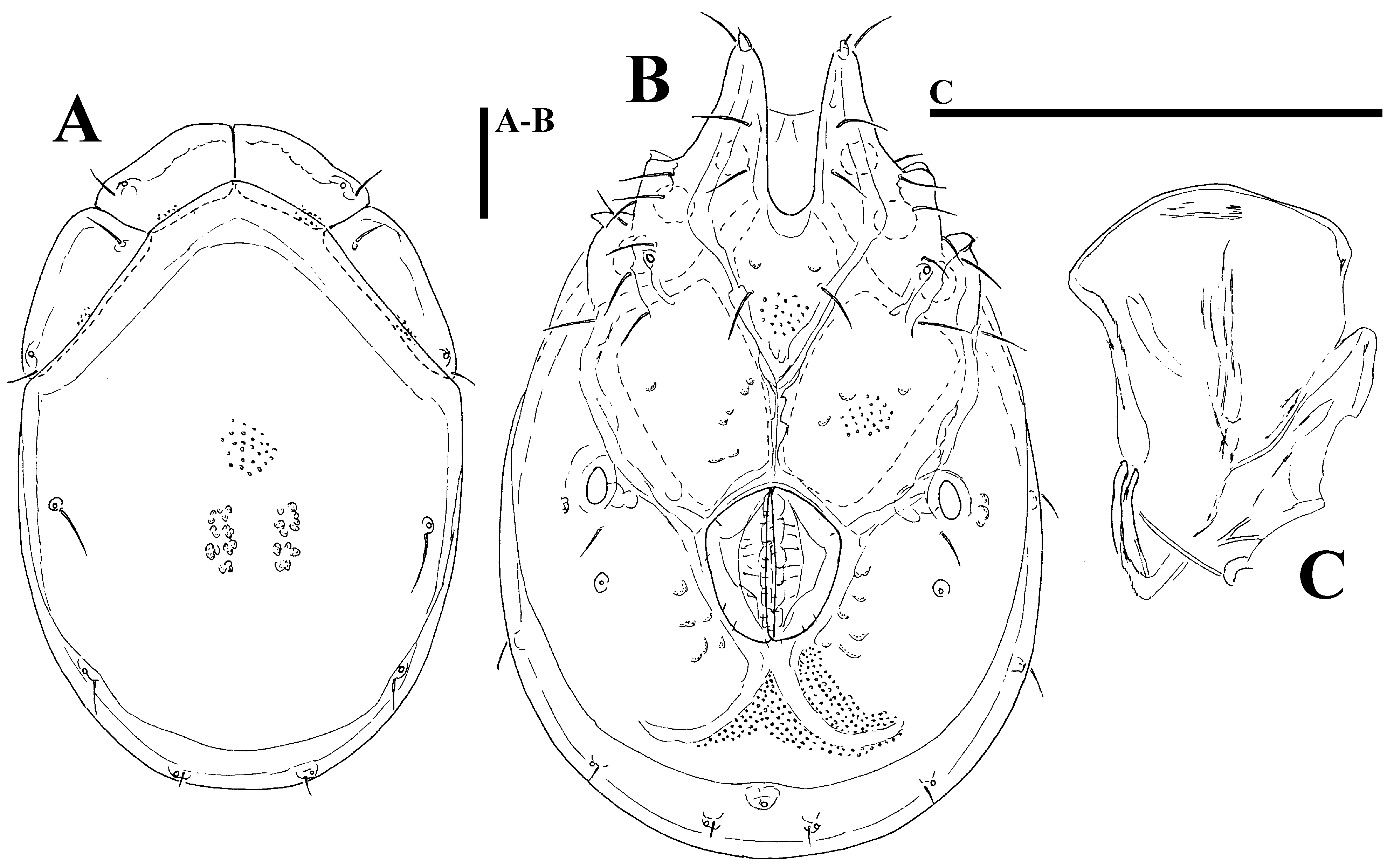
Morphology. General features —Idiosoma elongated oval; frontal platelets only slightly shorter than shoulder platelets; frontal margin medially straight or slightly convex, between flat, anterolaterally pointed protrusions ( Figs. 39A View FIGURE 39 A – G , 40A View FIGURE 40 A – G ); Cxgl–4 located far anteriorly, near tips of Cx-I; gnathosomal bay moderately deep, U-shaped, proximally rectangular box-shaped; suture line of Cx-IV strongly extending posteriorly beyond posterior margin of genital field, laterally curved ( Figs. 39C View FIGURE 39 A – G , 40C View FIGURE 40 A – G ); excretory pore and Vgl–2 away from the line of primary sclerotization, excretory pore anterior to Vgl–2; ventral seta on P-4 short ( Figs. 39D–E View FIGURE 39 A – G , 40D–E View FIGURE 40 A – G ). Male: medial margin of Cx-II/III relatively short; genital field elongated, anteriorly enlarged. Female: genital field elongated pentagonal, anteriorly rounded.
......continued on the next page Apkonu Apkonu M. procursa, K.O. Viets & Böttger 1974 , Congo
stream, ♂, stream, ♀, holotype ♀ paratype ♂, ♂, n = 8 ♀, n = 9
n = 5 n = 2 slide no. 4768 slide no. 4797
......continued on the next page Remarks. There appear to be two forms of this species (or two sibling species within Monatractides microstoma species - complex, see above for a discussion). At one extreme end (Form ''A''), specimens are generally of smaller dimensions, the dorsal plate with the area of primary sclerotization bears four dorsoglandularia ( Figs. 39B View FIGURE 39 A – G , 41A View FIGURE 41 A – D , 43A View FIGURE 43 A – I ), the gnathosoma is more elongated ( Fig. 39F View FIGURE 39 A – G ) and the male genital field is anteriorly projecting and strongly pointed ( Figs. 39C View FIGURE 39 A – G , 42A View FIGURE 42 A – K. A – H ). At the other extreme end (form ''B''), specimens are generally of larger dimensions, the dorsal plate with the area of primary sclerotization bears two dorsoglandularia ( Figs. 40A View FIGURE 40 A – G , 41C View FIGURE 41 A – D , 43B View FIGURE 43 A – I ), the gnathosoma is more triangular in shape ( Fig. 40F View FIGURE 40 A – G ), P-4 has a small but clearly visible denticle near insertion of ventral setae ( Figs. 40D–E View FIGURE 40 A – G ) and the male genital field is anteriorly less projecting and slightly pointed ( Figs. 40C View FIGURE 40 A – G , 42C–D View FIGURE 42 A – K. A – H ). The figures 39 A–G and 40 A–G illustrate the two extremes. However it is necessary to stress that in many cases we observed intermediate characters (in shape of male genital field, see Figs. 42A–D View FIGURE 42 A – K. A – H ) between these two extremes, even though at most sampling sites where these two forms are living together it is easily possible to identify the two groups (see ''New records'').
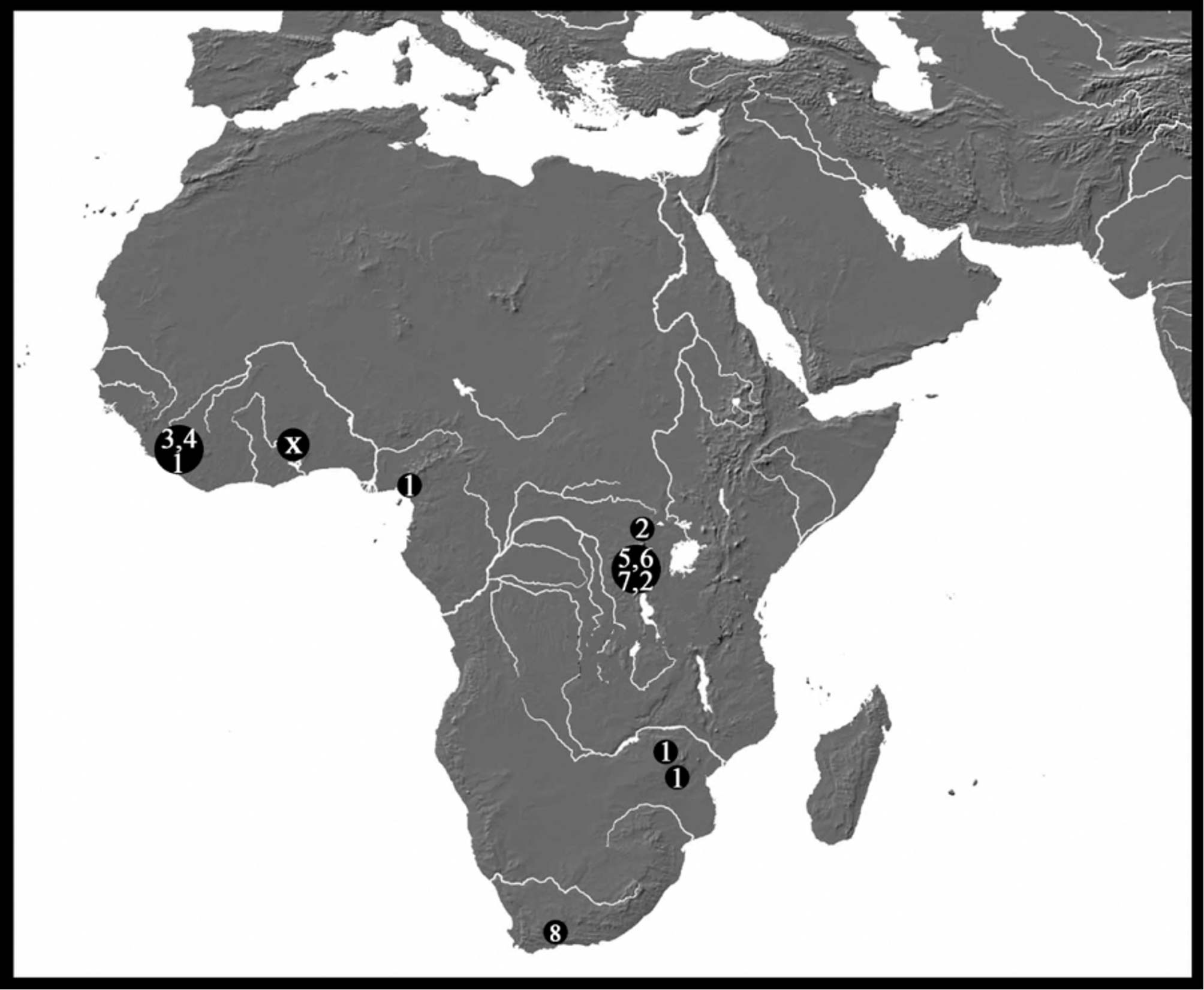
There is a large group of African species sharing the more or less elongated idiosoma and the box-shaped gnathosomal bay. This group includes several similar but still weakly defined species, i.e., Monatractides microstoma ( Koenike, 1898) , M. amplipalpis (Lundblad, 1951) , M. longicoxalis ( Cook, 1966) , M. dolichosoma ( Cook, 1966) , M. congoensis ( K.O. Viets, 1974) , M. rivalis ( K.O. Viets, 1974) , M. procursa ( K.O. Viets, 1974) and M. nigromontanus Goldschmidt & Smit 2009 (see Fig. 45 View FIGURE 45 for their distribution in Africa).
Due to the similar shape of the idiosoma and palp the specimens from our study most closely resemble Monatractides microstoma ( Koenike, 1898) and M. procursa ( K.O. Viets, 1974) . The latter species was described by K.O. Viets (in K.O. Viets & Böttger (1974) from Congo ( Zaire). Due to the dorsal plate with the area of primary sclerotization bearing two dorsoglandularia and similar shape of the genital field, M. procursa resembles more the specimens of Form ''B'' in our study. Differences with figures and measurements in the original description are found in the excretory pore shifted to the line of primary sclerotization in the female (see Fig. 75 in K.O. Viets & Böttger 1974, postgenital area in male not illustrated).
Monatractides microstoma was decribed by Koenike (1898) from Cameroon based on a single female. Later on a detailed description of the female (from Manoka near Duala, prep. no. 1390) was given by K. Viets (1958). Cook (1966) reported M. microstoma as the most frequently collected and most widely distributed torrenticolid species in Liberia and provided illustrations and descriptions of the male. K.O. Viets (1971) published from Zimbabwe a female slightly larger than those from Liberia also under the name microstoma . Due to the dorsal plate with the area of primary sclerotization bearing four dorsoglandularia, M. microstoma is more similar to the specimens of Form ''A'' of our study. Males from Liberia differ from those in our study in a medial suture of Cx- II+III of moderate length, the genital field anteriorly less projecting and slightly pointed and a less extended postgenital area (see Cook 1966, fig. 262). Among the specimens from Ghana we found a single male from Tagbo stream which fits well Cook’s (1966) description of microstoma (see Figs. 46A–C View FIGURE 46 A – C , and measurements in Tab. 4 View TABLE 4 ). This specimen also agrees in the shape of the dorsal shield, gnathosoma elongated in lateral view and P-4 without lacking denticle at insertion of ventral setae, but it is notably larger than our specimens from Ghana.
As the holotype of M. microstoma is lost (see K. Viets 1958), additional material, including the male, and selection of a neotype from the locus typicus is necessary to clarify the taxonomy of this species.
Monatractides rivalis ( Congo) and M. nigromontanus ( South Africa) are two other species similar in having an elongated genital field, a box-shaped gnathosomal bay and a short medial margin of Cx-II+III. The latter species differs in a more elongated idiosoma in both sexes, a more trapezoid shape of the genital field, anteriorly less enlarged and slightly pointed, and a sligtly longer medial margin of Cx-II+III in the male (see Goldschmidt & Smit 2009). Monatractides rivalis can be distinguished by the larger dimensions in both sexes and a characteristically rounded lateral margin Cx-II (see K.O. Viets & Böttger 1974).
For the time being we keep the populations from Ghana not assigned to either M. microstoma or M. procursa , mainly because the unclear taxonomy of M. microstoma . Understanding the taxonomic position of the M. microstoma - complex is not possible without additional material from a wide area and/or without the application of molecular techniques.
Ejaculatory complex. In male specimens of form ''A'' the ejaculatory complex was not found but a sclerotized framework immediately above the genital field can be hardly visible (see Figs. 44E–G View FIGURE 44 A – L ). In some examined males of form “B” the ejaculatory complex was reduced to a sclerotized framework ( Fig. 44H View FIGURE 44 A – L ) as in the previous form. However, in other specimens of form ''B'' a genital skeleton with more or less developed series of membranous chambers attached to a sclerotized framework can be observed (see Figs. 44I –L View FIGURE 44 A – L , 46C View FIGURE 46 A – C ). This ejaculatory organ was shortened apically, the proximal chamber is large, the proximal and distal arms are small, the carina anterior is not developed and the apical setae is very long ( Figs. 42E–G View FIGURE 42 A – K. A – H ). Figures 42G–H View FIGURE 42 A – K. A – H illustrate the ejaculatory complex found in the largest specimens of form ''B'', possibly the last stage in the development of the ejaculatory complex.
We considered several possibilities: 1) it is possible that populations (or species?) with developed or reduced ejaculatory complex exist? 2), it is possible that populations (or species?) exist in which the coalescence of membranous chambers to make up a normally developed ejaculatory complex is more or less progressed and 3), the ejaculatory complex may be more or less developed in animals of different ages. At the time being we think that differences in development of ejaculatory complex might be the result of growth.
No ejaculatory complex has been described for Monatractides microstoma , nor for M. procursa . A very unusually shaped ejaculatory complex was found in Monatractides jucundus (see Fig. 42I View FIGURE 42 A – K. A – H , figure taken from Lundblad 1952). Figure 42K View FIGURE 42 A – K. A – H (taken from Lundblad 1952) shows the genital flap in one of Lundblad’s fixed specimen, with the apical part of the opened ejaculatory complex ending in two apical setae and a two-parted, apically pointed membranous structure, similar to condition found in ejaculatory complex of our specimens illustrated in Fig. 42G View FIGURE 42 A – K. A – H .
Malformation. In one male (form ''A'') from Kintampo Falls the anterolateral platelets of dorsal shield were asymetrically developed ( Fig. 43C View FIGURE 43 A – I ).
Habitat. Sandy/bouldary streams, shaded by riparian vegetation ( Figs. 53A–B, D View FIGURE 53 A – E ).
TABLE 4. Measurements of Monatractides microstoma (Koenike, 1898) — species complex (Form “ A “) from Ghana, and female of M. microstoma from Cameroon (from K. Viets 1958, prep. no. 1390; * calculated from figure).
| Tagbo River ♂, Plunge pool, ♂ Plunge pool, n = 2 2♀ | M. microstoma sensu Cook, 1966 , Tagbo River, ♂ | K. Viets, 1958 Cameroon, M. microstoma , prep. no. 1390, ♀ | |
|---|---|---|---|
| Idiosoma L Idiosoma W Idiosoma L/W ratio | 573–647 641 675–703 398–447 459 466–506 1.44–1.45 1.4 1.39–1.45 | 733 476 1.54 | 640 535 1.2 |
| Ds L Ds W Ds L/W ratio | 468–533 534 548–586 340–381 381 389–405 1.38–1.4 1.4 1.41–1.45 | 600 406 1.48 | 590 420 1.4 |
| Dp L Sh plate L Sh plate W | 436–491 497 513–545 109–131 122–123 125–127 42–69 50 45–55 | 550 169–172 68–73 | 525 145 - |
| Sh plate L/W F plate L F plate W | 1.9–2.6 2.4–2.5 2.3–2.8 108–122 125–127 116–125 45–56 53 50–55 | 2.3–2.5 142–144 72 | - 115 65 |
| F plate L/W ratio Sh pl L/f pl L ratio Gnathosomal bay L | 2.2–2.4 2.3–2.4 2.2–2.4 0.97–1.07 0.97–1.0 1.04–1.1 123–143 145 141–158 | 2.0 1.19–2.0 153 | 1.8 1.26 154 |
| Cx-I L Cx-I mL Cx-II+III mL | 209–241 247 236–253 85–97 102 95–96 25–31 23 29–34 | 313 159 83 | 260 - 40 |
| Cx-1 L/Cx-II+III mL Cx-I mL/Cx-II+III mL ratio Genital field L | 8.4–7.9 10.7 7.4–8.1 3.2–3.4 4.4 2.8–3.3 120–133 137 134–144 | 3.8 1.9 137 | 6.5 - 149 |
| Genital field W Gf L/W ratio Dist gf—expo | 101–111 111 120–127 1.19 1.23 1.12–1.14 156–181 186 175–194 | 116 1.18 144 | 133 1.12 - |
| Dist gf—cauda | 219–241 233 269–270 | 198 | - |
| Ec L Egg (maximum diameter) | - - - - - 169–178 | - - | - - |
| Gnathosoma vL Chelicera L Palp, total L | 148–160 152 160–164 165–189 - 192 148–163 172 163–176 | 192 211 182 | - 205 - |
| P-1 dL/H P-2 dL/H P-3 dL/H | 19–22/17–20 23/20 22–24/21–22 43–46/31–33 48/33 46–51/35 31–34/25–27 34/28 34–35/28 | 25/22 51/37 39/31 | - 53 33/26 |
| P-4 dL/H P-5 dL/H P-1 dL/H ratio | 38–43/18–19 46/18–19 43–46/18–19 17–19/9–11 21/10 18–20/10–12 1.08–1.1 1.16 1.04–1.12 | 46/24 21/12 1.14 | 45 13 - |
| P-2 dL/H ratio P-3 dL/H ratio P-4 dL/H ratio | 1.4–1.41 1.46 1.34–1.46 1.21–1.25 1.22 1.25 2.1–2.27 2.5 2.37–2.5 | 1.37 1.26 1.93 | 1.45* 1.27 2.3* |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
|
SubGenus |
Monatractides |
Monatractides urania
| Pešić, Vladimir & Smit, Harry 2014 |
M. nigromontanus
| Goldschmidt & Smit 2009 |
M. nigromontanus
| Goldschmidt & Smit 2009 |
M. procursa , K.O. Viets & Böttger 1974
| K.O. Viets & Bottger 1974 |
M. procursa
| K.O. Viets 1974 |
M. procursa , K.O. Viets & Böttger 1974
| K.O. Viets & Bottger 1974 |
M. congoensis (
| K.O. Viets 1974 |
M. rivalis (
| K.O. Viets 1974 |
M. procursa (
| K.O. Viets 1974 |
M. procursa (
| K.O. Viets 1974 |
M. congoensis
| K.O.Viets 1974 |
M. rivalis
| K.O.Viets 1974 |
M. procursa
| K.O.Viets 1974 |
M. microstoma sensu
| Cook 1966 |
M. microstoma sensu
| Cook 1966 |
M. longicoxalis (
| Cook 1966 |
M. dolichosoma (
| Cook 1966 |
M. longicoxalis
| Cook 1966 |
M. dolichosoma
| Cook 1966 |
Monatractides convexiscutata
| K. Viets 1958 |
M. amplipalpis
| Lundblad 1951 |
M. amplipalpis
| Lundblad 1951 |
Monatractides jucundus
| Lundblad 1927 |
M. cf. ventriosus
| K. Viets 1916 |
Monatractides microstoma (
| Koenike 1898 |
Monatractides microstoma
| Koenike 1898 |
Monatractides microstoma
| Koenike 1898 |
Monatractides microstoma (
| Koenike 1898 |
Monatractides microstoma (
| Koenike 1898 |
M. microstoma
| Koenike 1898 |