Fizesereneia daidai Zayasu
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3681.3.5 |
|
publication LSID |
lsid:zoobank.org:pub:EC1D68B4-D121-4D4F-9D0E-52EA59B0C59A |
|
DOI |
https://doi.org/10.5281/zenodo.6163801 |
|
persistent identifier |
https://treatment.plazi.org/id/62A4B158-0820-4BAA-AE0D-A73A7718BA2A |
|
taxon LSID |
lsid:zoobank.org:act:62A4B158-0820-4BAA-AE0D-A73A7718BA2A |
|
treatment provided by |
Plazi |
|
scientific name |
Fizesereneia daidai Zayasu |
| status |
sp. nov. |
Fizesereneia daidai Zayasu View in CoL sp. nov.
[Japanese name: Daidai-kubomi-sangoyadorigani] Figs. 2–8 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8
Type specimens. Holotype: 1 ovig. female, 2.4 m, Kanayama Bay (33.690°N 135.336°E), Shirahama-cho, Nishimuro-gun, Wakayama Pref., Kii Peninsula, Japan, 5 Sept. 2012, NSMT-Cr 22340 (5.2 × 4.1 mm). Paratypes: 1 male, 1.7 m, Sabiura (33.480°N 135.745°E), Kushimoto-cho, Higashimuro-gun, Wakayama Pref., Kii Peninsula, Japan, 22 Oct. 2012, NSMT-Cr 22341 (3.5 × 2.6 mm). 1 female, 2.2 m, same locality as NSMT-Cr 22341, May 24 2011, QM-W29180 (4.7 × 4.0 mm). 1 ovig. female, 1 female, same data as QM-W29180, SMBL Type 459 (4.2 × 3.3 mm, 5.0 × 4.0 mm). 1 ovig. female, 2.9 m, Kopepe Beach (27.068°N 142.191°E), Chichijima, Ogasawara Is., Japan, 13 June 2011, SMBL Type 460 (4.0 × 3.1 mm). 1 female, 1.7 m, same locality as NSMT-Cr 22341, Oct. 22, 2012, QM-W29181 (5.3 × 4.8 mm). 1 ovig. female, same data as QM-W29181, USNM 1205403 (5.4 × 4.4 mm). 1 female, same data as QM-W029181, AM P90345 (5.5 × 4.2 mm). 1 ovig. female, same data as QM-W029181, AM P90352 (5.7 × 4.2 mm). 2 ovig. females, same data as QM-W29181, BMNH (5.8 × 4.8 mm, 5.4 × 4.3 mm). 1 ovig. female, same data as NSMT-Cr 22341, USNM 1205404 (5.6 × 4.6 mm). 2 females, 2.5 m, same locality as NSMT- Cr 22341, Oct. 22, 2012, NSMT-Cr 22342 (5.5 × 4.7 mm, 4.4 × 3.5 mm).
Other materials. 1 ovig. female, 2 m, same locality as NSMT-Cr 22341, 21 May 2010, NSMT-Cr 22343 (5.0 × 3.8 mm). 1 ovig. female, same data as NSMT-Cr 22343, NSMT-Cr 22344 (3.8 × 3.3 mm). 1 ovig. female, same data as SMBL Type 460, NSMT-Cr 22345 (4.5 × 3.3 mm). All specimens were collected by the first author from Micromussa amakusensis , except SMBL Type 460 and NSMT-Cr 22345, which were collected from Micromussa sp.
Comparative material examined. Fizesereneia ishikawai Takeda & Tamura 1980 : holotype, female, 5 m, Arakawa, Ishigakijima, Yaeyama Group, Ryukyu Is., Okinawa Pref., Japan, 24 Apr. 1979, coll. Yôichi Tamura, host coral Symphyllia sp., NSMT-Cr 6340 (4.4 × 3.9 mm).
Fizesereneia stimpsoni (Fize & Serène 1955b): 1 ovig. female, 2 females 1.5 m, Yaene, Hachijojima, Izu Is., Japan, 3 May 1980, coll. Y. Tamura, host coral Acanthastrea sp., NSMT-Cr 9009. 1 female, 10 m, same locality as NSMT-9009, 3 May 1980, coll. Y. Tamura, host coral Acanthastrea sp., NSMT-Cr 13696. 1 female, 15 m, same locality as NSMT-9009, 2 May 1980, coll. Y. Tamura, host coral Acanthastrea sp., NSMT-Cr 13697. 3 ovig. females, 5 m, Kanayama Bay, Shirahama-cho, Nishimuro-gun, Wakayama Pref., Kii Peninsula, Japan, 21 Apr. 2010, host coral Acanthastrea hemprichii (Ehrenberg, 1834) , NSMT-Cr 22346 (5.8 × 4.1 mm, 4.9 × 3.6 mm, 4.7 × 3.8 mm). 1 female, Sabiura, Kushimoto-cho, Higashimuro-gun, Kii Peninsula, Wakayama Pref., Japan, 21 May 2010, host coral Acanthastrea hemprichii , NSMT-Cr 22347 (6.4 × 5.1 mm). 1 ovig. female, same data as NSMT-Cr 22347, NSMT-Cr 22348 (5.4 × 4.4 mm). 1 ovig. female, Nanatsuyama Beach, Nakanoshima, Tokara Isls., Kagoshima-gun, Kagoshima Pref., Japan, 4 July 2010, host coral Acanthastrea echinata (Dana, 1846) , NSMT-Cr 22349 (5.0 × 3.8 mm).
Fizesereneia latisella Kropp 1994: 1 View in CoL ovig. female, 15 m, Kuroshima, Yaeyama Group, Ryukyu Is., Okinawa Pref., Japan, 22 Apr. 1979, coll. Y. Tamura, host coral Lobophyllia hemprichii (Ehrenberg, 1834) , NSMT-Cr 6336 (5.1 × 4.6 mm). 2 ovig. females, 15 m, Kohamajima, Yaeyama Group, Ryukyu Is., Okinawa Pref., Japan, Apr. 23 1979, coll. Y. Tamura, host coral Lobophyllia hemprichii , NSMT-Cr 6337 (5.2 × 4.7 mm, 5.0 × 4.5 mm). 1 male, 1 female, Kaminatomae, Hachijojima, Izu Is., Japan, 31 Oct. 1981, coll. Kônosuke Takahashi, host coral Lobophyllia sp., NSMT-Cr 9007. 2 females, same locality as NSMT-Cr 9007, 15 Aug. 1981, coll. K. Takahashi, host coral Lobophyllia sp., NSMT-Cr 9008. 2 ovig. females, 1 male, 3.5 m, Kopepe Beach, Chichijima, Ogasawara Is., Japan, 14 June 2011, host coral Symphyllia radians, Milne Edwards & Haime, 1849 , NSMT-Cr 22350 (5.7 × 5.3 mm, 5.7 × 4.9 mm, 4.4 × 3.8 mm). 1 ovig. female, 1.8 m, Sesokojima, Motobu-cho, Kunigami-gun, Okinawa Pref., Japan, 13 Sept. 2010, host coral Symphyllia recta (Dana, 1846) , NSMT-Cr 22351 (6.5 × 5.7 mm). 1 ovig. female, 3.6 m, same locality as NSMT-Cr 22352, 12 Sept. 2010, host coral Symphyllia sp., NSMT-Cr 22352 (5.8 × 5.7 mm).
Fizesereneia heimi View in CoL (Fize & Serène 1955a): 1 ovig. female, Sabiura, Kushimoto-cho, Higashimuro-gun, Wakayama Pref., Kii Peninsula, Japan, 21 May 2010, coll. Hironobu Fukami, host coral not recorded, NSMT-Cr 22353 (8.5 × 7.6 mm). 1 ovig. female, 1.8 m, Sesokojima, Motobu-cho, Kunigami-gun, Okinawa Pref., Japan, 13 Sept. 2010, host coral Symphyllia radians , NSMT-Cr 22354 (5.2 × 5.2 mm). 1 male, 1 ovig. female, 1 female, 15 m, Higashihennazaki, Miyako-shi, Okinawa Pref., Japan, 21 Aug. 2010, host coral Symphyllia recta , NSMT-Cr 22355 (4.5 × 3.7 mm, 6.2 × 4.8mm, 6.0 × 4.9 mm). 1 ovig. female, Nanatsuyama Beach, Nakanoshima, Tokara Is, Kagoshima-gun, Kagoshima Pref., Japan, 6 July 2010, host coral Symphyllia recta , NSMT-Cr 22356 (5.3 × 4.3 mm). 1 male, 1.8 m, Sesokojima, Motobu-cho, Kunigami-gun, Okinawa Pref., Japan, 12 Sept. 2010, host coral Symphyllia recta , NSMT-Cr 22357 (4.5 × 3.6 mm). 1 female, 1.6 m, same locality as NSMT-Cr 22357, 12 Sept. 2010, host coral Lobophyllia corymbosa (Forskål, 1775) , NSMT-Cr 22358 (3.5 × 3.0 mm). Specimens were collected by the first author unless specified otherwise.
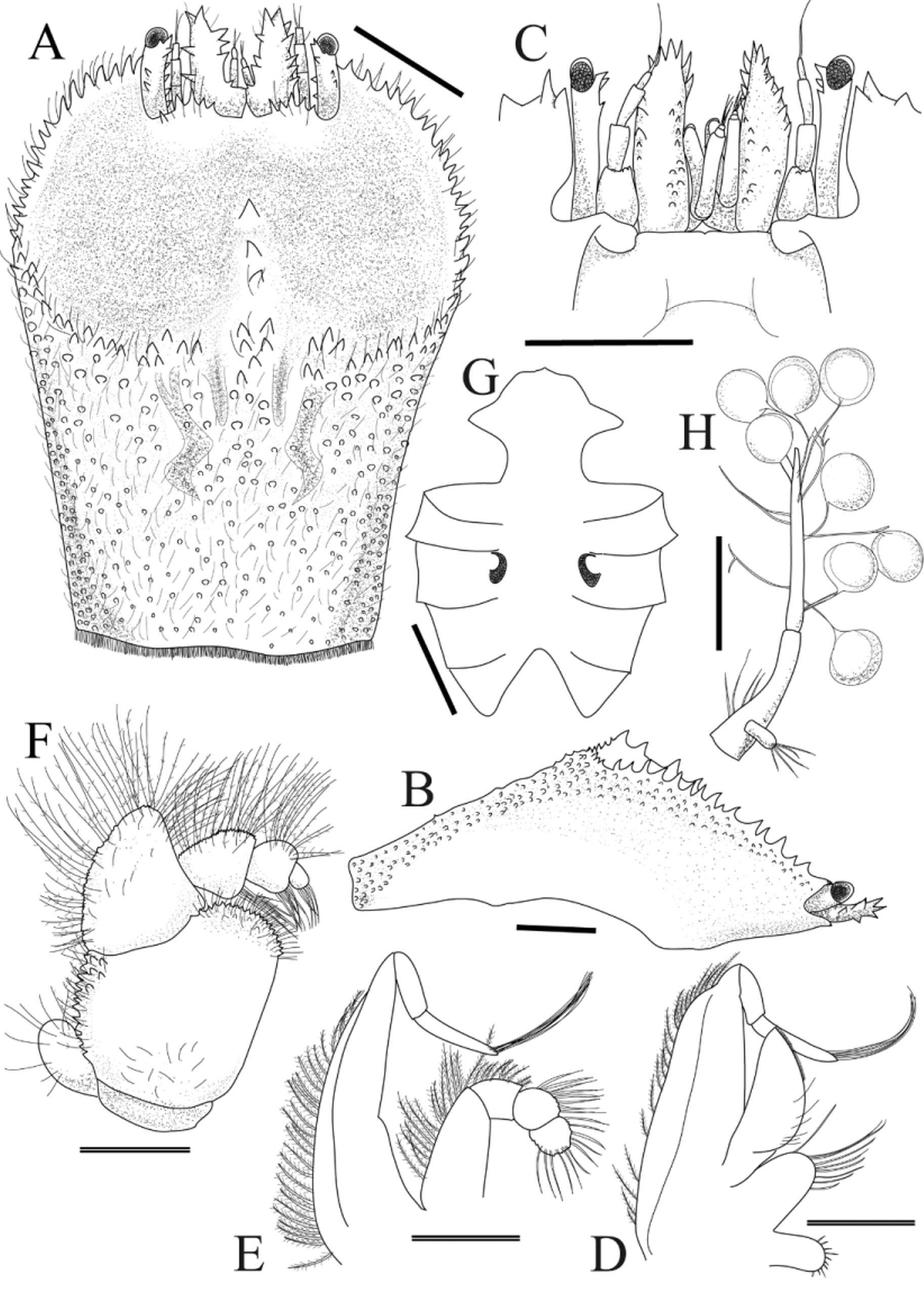
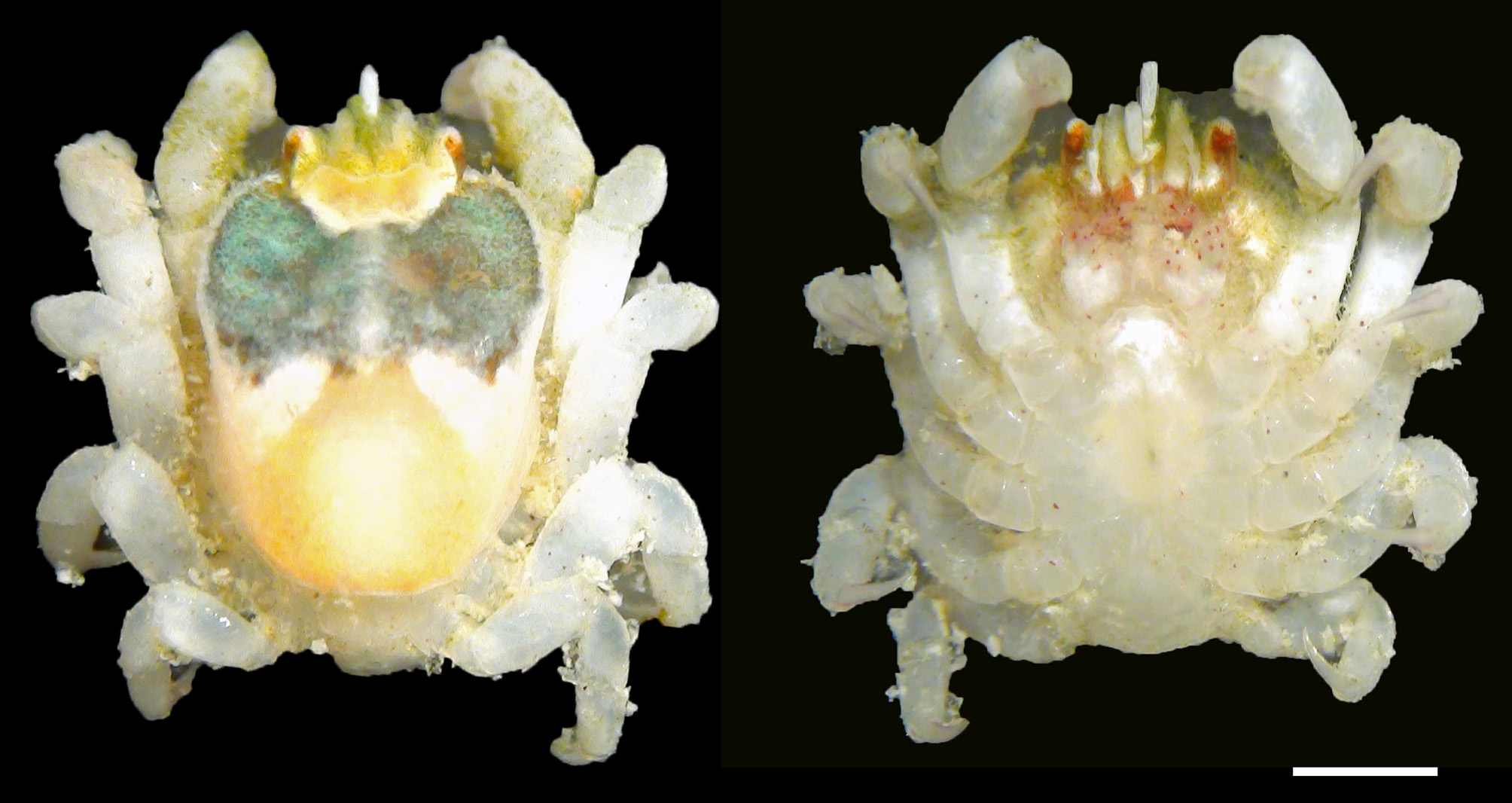
Description of holotype (female). Carapace ( Fig. 2 View FIGURE 2 A) longer than broad, CL 1.4 times longer than CW; dorsal surface strongly convex in lateral view ( Fig. 2 View FIGURE 2 B), deflected anteriorly, with angle between anterior half, posterior half of carapace approximately 60°, anterior half with large depression. Greatest width of carapace where posterior margin of depression meets lateral margin of carapace. Frontal margin armed with 5 anteriorly directed spines on convex lobe on either side of spiny rostrum, fringe of setae; external orbital angle upswept, far exceeding level of frontal margin, continuing to anterolateral margin; distance between external orbital angles about three-fifths breadth of posterior margin of carapace, about two-fifths breadth of greatest width of carapace. Anterior half of anterior carapace depression smooth, flat; posterior half of depression smooth, divided incompletely into 2 concavities by median longitudinal ridge armed with 3 spines, originating from posterior margin of depression; margin of depression with long plumose setae, upturned spines; posterior margin of depression almost parallel to posterior margin of carapace. Posterior half of dorsum covered with numerous short setae, granules; cardiointestinal region slightly outlined by shallow furrow; posterior margin of carapace fringed with dense, short plumose setae. Pterygostomial region completely fused to carapace.
Anterior margin of epistome ( Fig. 2 View FIGURE 2 C) almost straight. Gonopore (vulva) ( Fig. 2 View FIGURE 2 G) elliptical to subrhombic, with small sternal vulvar cover.
Basal segment of antennule ( Fig. 2 View FIGURE 2 C) reaching beyond external orbital angle, corneae; lateral, mesial surfaces armed with several spinules, setae. Ocular peduncles mostly exposed in dorsal view, with several spinules on dorsal, mesial surfaces; corneae elliptical, slightly longer than broad.
Exopod of MXP-1 ( Fig. 2 View FIGURE 2 D) elongate trapezoidal shape, fringed with plumose setae; endopod semi-ellipsoidal, with sparse setae; protopod semi-oval, with sparse setae.
Exopod of MXP-2 ( Fig. 2 View FIGURE 2 E) elongated triangular, lateral margin with plumose setae; lateral margin of merus, carpus of endopod with plumose setae; propodus, dactyl with setae.
MXP-3 ( Fig. 2 View FIGURE 2 F) surface flat; exopod subcircular, reaching half length of ischium, with some setae; ischium smooth, with scattered setae; mesial margin straight, anteromesial lobe finely denticulate; lateral margin with finely tuberculated projections, plumose setae; lateral margin of merus with long plumose setae; distal portions of carpus, propodus, dactylus each with bundle of thick setae medially; lateral margin of 3 segments covered with dense, long plumose setae.
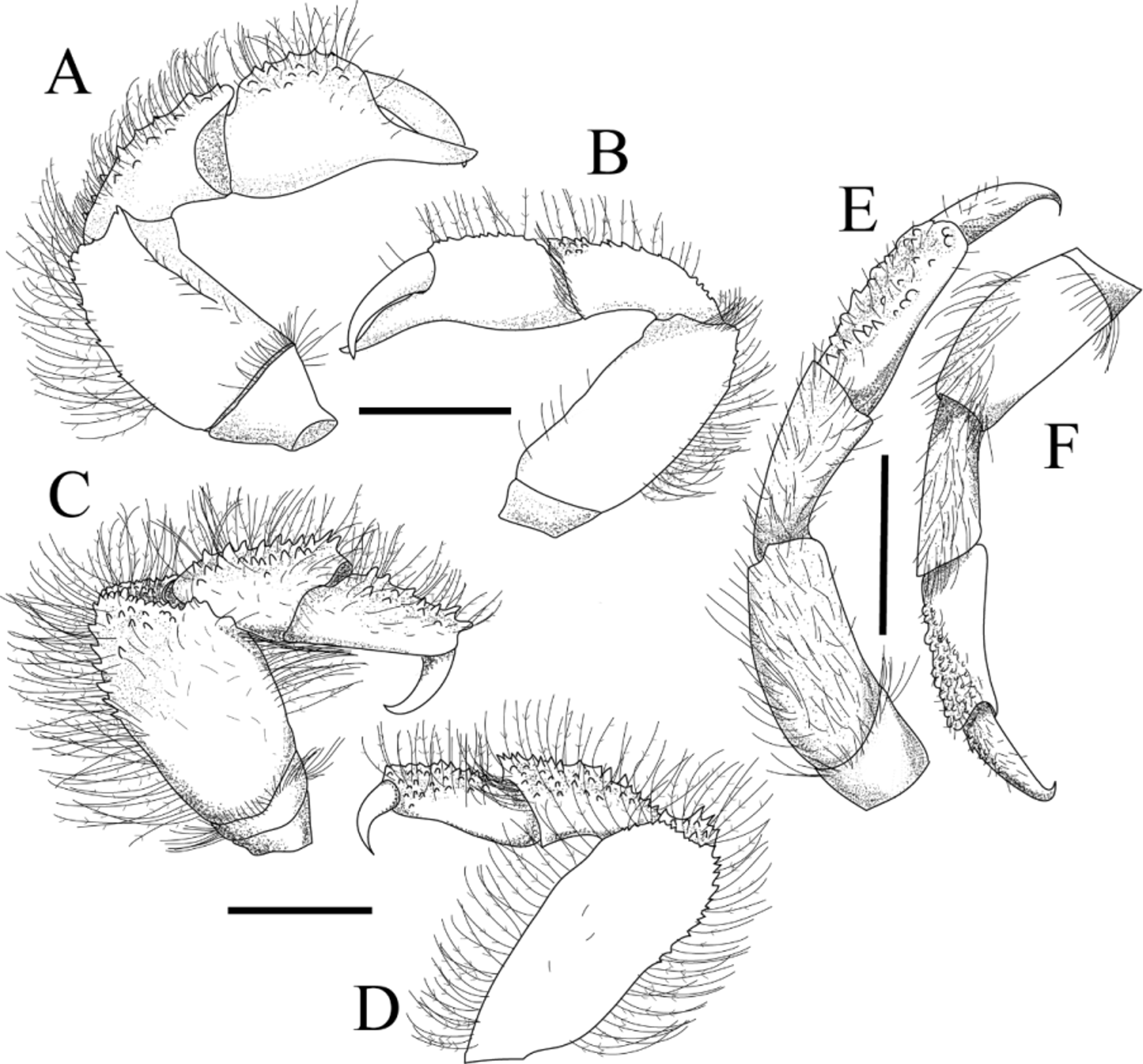
Chelipeds (P-1) ( Fig. 3 View FIGURE 3 A, B) slender, equal, compressed dorsoventrally, hidden under carapace in dorsal view alive; merus length twice height, dorsal surface with long plumose setae, tubercles; carpus length about twice height; palm, carpus with dorsal surfaces tuberculate, setae; tips of fingers slightly crossing; fingers slender, as long as palm, mesial surfaces of fingers smooth.
P-2 ( Fig. 3 View FIGURE 3 C, D) longer, stouter than P-1; distal margin of ischium fringed with setae; merus flaring distally, with prominent distomesial expansion, dorsal surface with conical spines on distal half; tubercles, plumose long setae distally; lateral surface covered with sparse, simple short setae, joint between merus, carpus not extending more than at right angle; carpus triangular in cross-section, ventral surface smooth, dorsal surface with several conical-shaped spines, plumose long setae; propodus as long as carpus, ventral surface smooth, dorsal surface covered by spinules, plumose long setae; dactylus half-length of propodus, smooth, sharp, curved ventrally.
P-3, P-4 similar to P-2, decreasing in size from P-2 to P-4, dorsal surfaces with tubercles instead of conicalshaped spines. P-5 ( Fig. 3 View FIGURE 3 E, F) slender, shorter than P-1, longer than P-3; merus with sparse, long plumose setae, dense setae at distal margin; dorsal surface of carpus with long plumose setae, minuscule tubercles; dorsal surfaces of propodus, dactylus with simple setae minute tubercles; dactylus curved ventrally.
Abdomen enlarged, lateral margin fringed with setae, thicker setae on somites 1–3. Abdomen with three pairs of pleopods; somite 2 with pair of biramous pleopods with rudimentary exopod ( Fig. 2 View FIGURE 2 H); somites 3?4 each with pair of uniramous pleopods. Egg size (in alcohol) 0.55 mm maximum diameter.
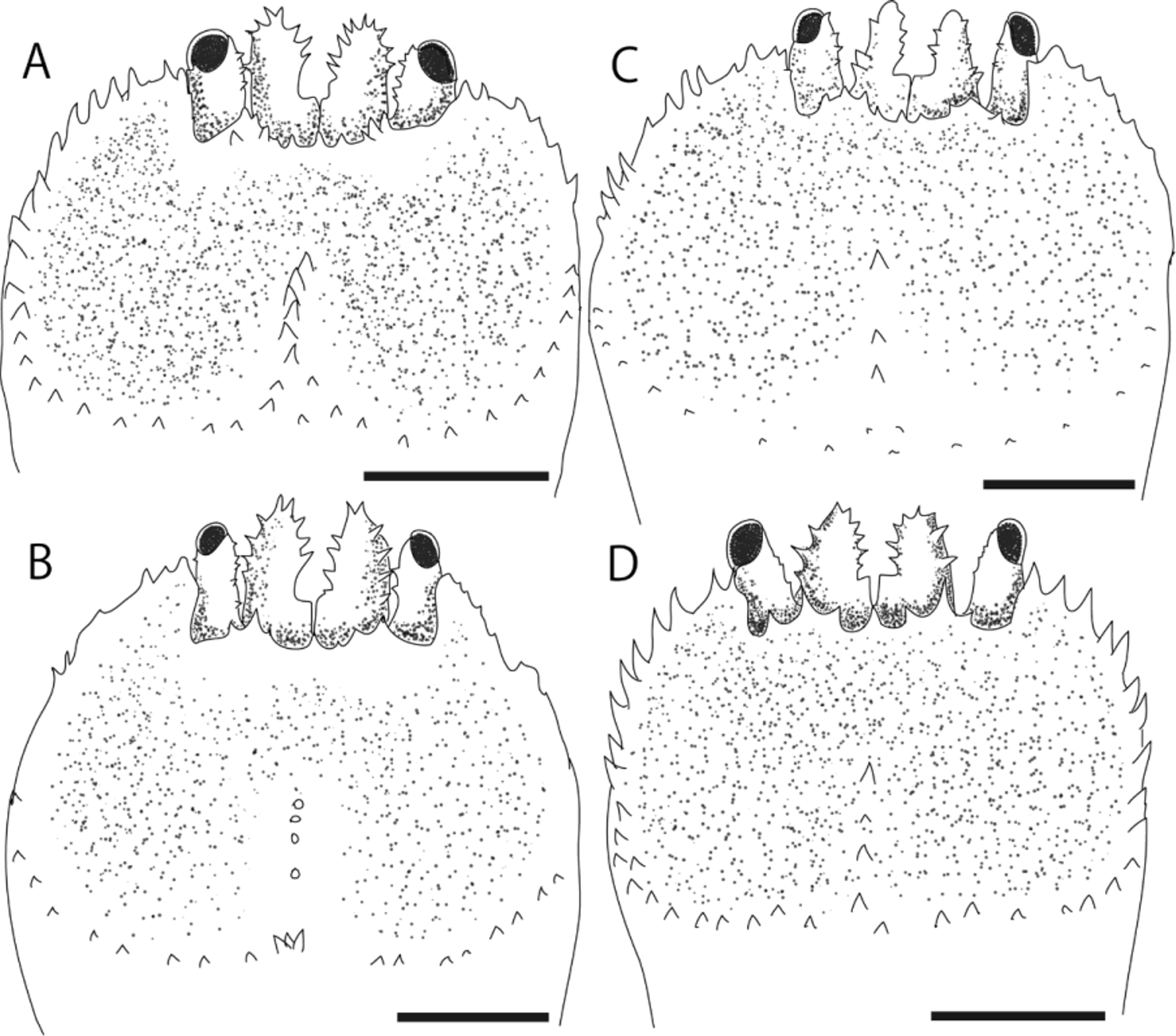
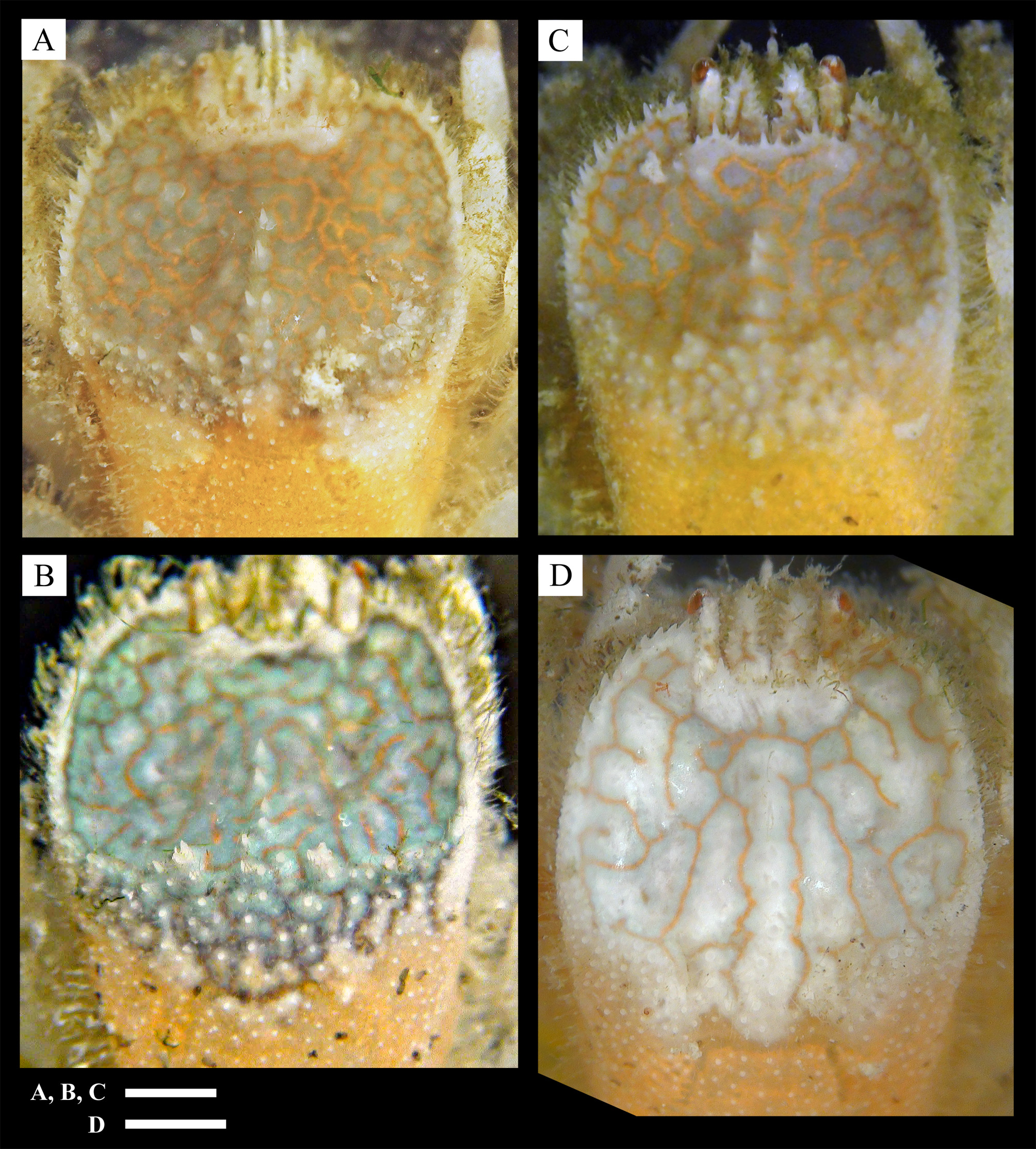
Variation. Considerable variation was shown in the number and size of spines on the margin of the anterior depression ( Fig. 4 View FIGURE 4 ). Numerous strong spines are present on the entire margin of the anterior carapace depression in the holotype ( Fig. 2 View FIGURE 2 A) and the paratype (SMBL Type 460; Fig. 4 View FIGURE 4 D), whereas spines are few and very weak in one of the paratypes (QM-W29180; Fig. 4 View FIGURE 4 B).
The spines on the midline of the anterior carapace depression also vary in number (0–6), acuteness (sharp to blunt-tipped) and size. This is also a reflection of the depth of the concavities on the posterior half of the anterior carapace depression. Of the paratype (SMBL Type 459 ovigerous one), two deep concavities are separated by a median longitudinal ridge armed with a row of spines ( Fig. 4 View FIGURE 4 A), whereas in a second paratype (QM-W29180), the region is rather flat and the median longitudinal ridge is barely distinguishable ( Fig. 4 View FIGURE 4 B).
Spines on the convex lobe of the frontal margin of the carapace may vary from 3–5, range in form from sharp denticles to broad spines ( Fig. 4 View FIGURE 4 A–D). Spines on the basal segment of the antennule may also vary in number and size.
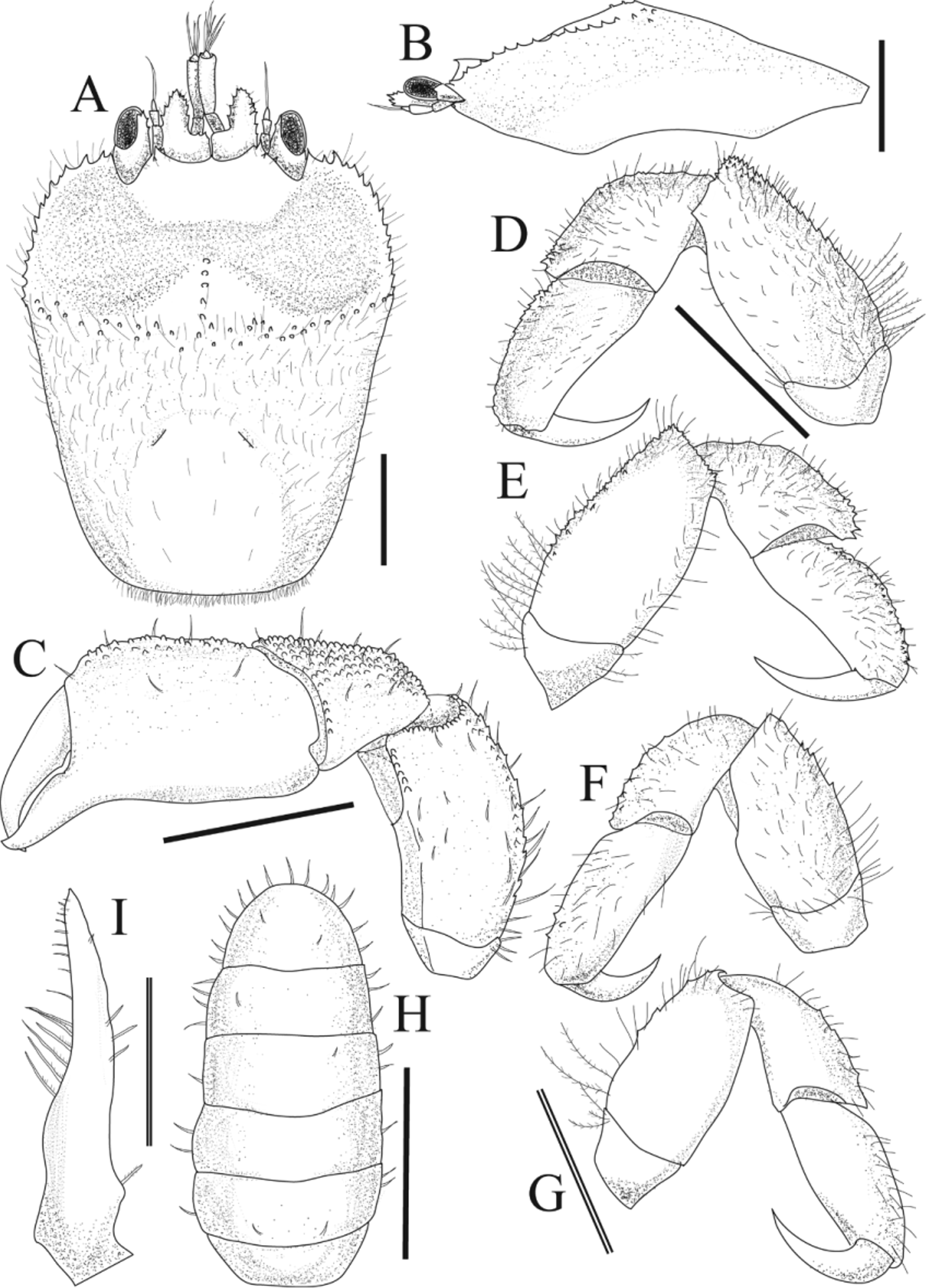
Description of Male (NSMT-Cr 22341). Generally similar to female, but much smaller and armature generally weaker ( Figs. 5 View FIGURE 5 , 6 View FIGURE 6 , 8 View FIGURE 8 A-a). Carapace ( Fig. 5 View FIGURE 5 A) longer than broad; posterior half of dorsum smoother than that in female. Chelipeds ( Fig. 5 View FIGURE 5 C) visible in dorsal view, proportionally larger than those in female; palm almost as large as female; carpus and merus shorter and more tuberculate. Telson broadly rounded ( Fig. 5 View FIGURE 5 H). G1 ( Fig. 5 View FIGURE 5 I) slightly curved, tapering, apex pointed.
Coloration. Figure 7 View FIGURE 7 shows color and pattern variation in females, all collected from the same host coral colony. When alive, the anterior carapace depression shows a finely to broadly reticulated pattern of orange on predominantly dark green or predominantly white background ( Figs. 7 View FIGURE 7 , 8 View FIGURE 8 A–b, A–c, B); posterior margin of depression brown or white; spines on carapace whitish; posterior half of carapace uniform light orange. Lateral half of ocular peduncles orange, antennules and all pereiopods whitish, palm of chelipeds with scattered red spots, dactyli of pereiopods with dark red spots.
In male ( Fig. 6 View FIGURE 6 ), the anterior depression on the carapace dorsum is greenish, with whitish margin; posterior half of carapace light orange. All pereiopods and MXP-3 are whitish, with scattered red spots.
Etymology. The specific name refers to the characteristic orange to light orange color of the new species when alive, especially on the posterior half of the carapace ( Figs. 6 View FIGURE 6 , 7 View FIGURE 7 , 8 View FIGURE 8 B), daidai meaning “orange color” in Japanese.
Coral host. The new species inhabits cylindrical cavities (pits) found within the scleractinian coral Micromussa amakusensis , and an unidentified Micromussa species. This genus is a new host record for Cryptochiridae .
Distribution. Currently known only from Wakayama Pref. Kii Peninsula, central Honshu, and Chichijima, Ogasawara Islands, Japan ( Fig. 1 View FIGURE 1 ).
Remarks. There are some clear differences between the new species and its congeners. Fizesereneia daidai sp. nov. has an orange colored posterior carapace ( Figs. 7 View FIGURE 7 , 8 View FIGURE 8 B), while the posterior carapaces of F. h e i m i, F. stimpsoni , F. i s h i k a w a i, and F. latisella are all whitish although the live coloration of F. tholia is unknown.
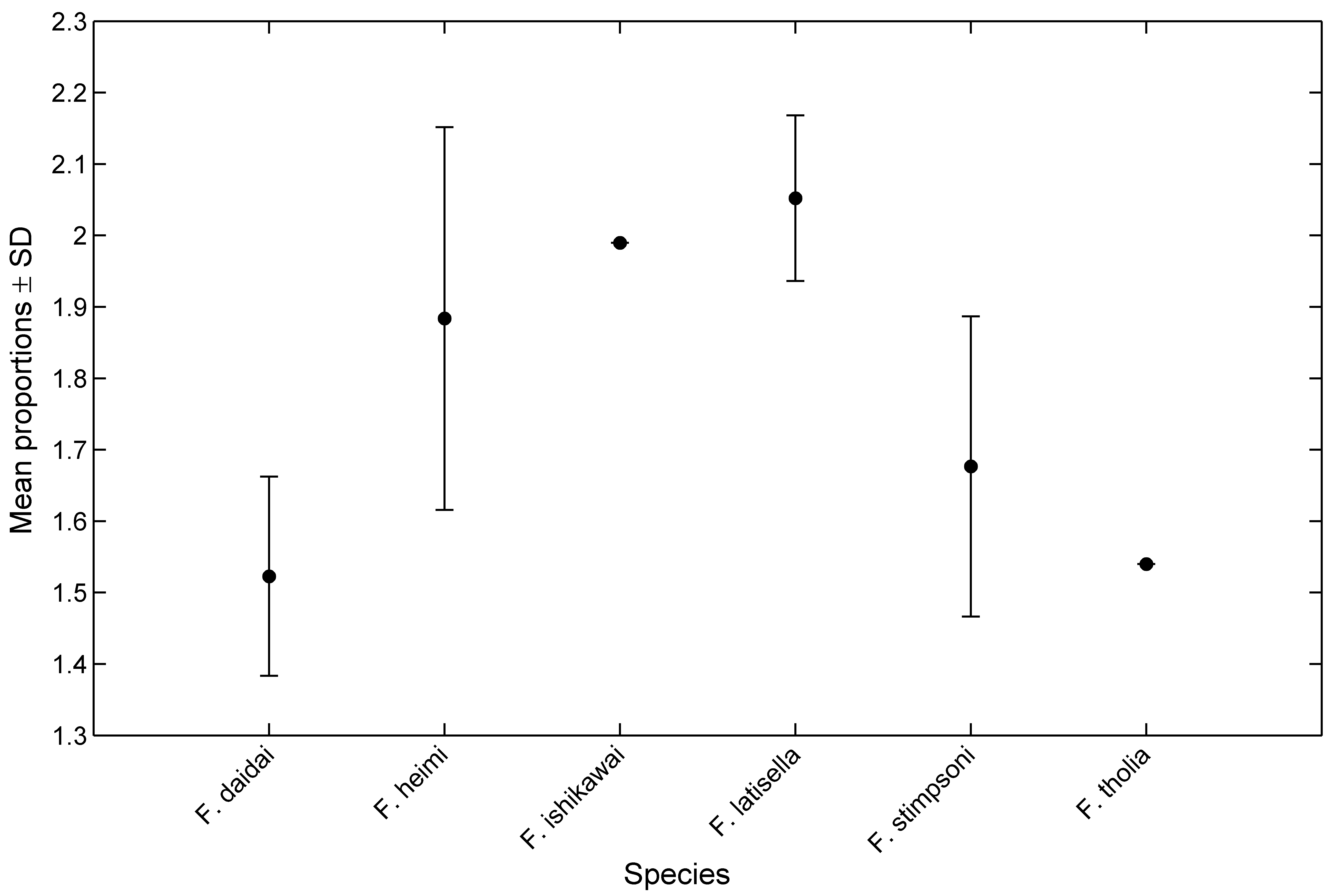
The carapace proportions of the new species also differ from other congeners; the carapaces of F. stimpsoni and F. i s h i k a w a i are subquadrangular, being widest across the anterior margin and narrower posteriorly (Fize & Serène 1955, Takeda & Tamura 1980). The carapaces are roughly hexagonal in F. h e i m i and F. latisella ( Takeda & Tamura 1980, 1983, Kropp 1994), with the widest part near the middle of the lateral margin. In contrast, the carapace of F. daidai sp. nov. and F. tholia are subrectangular, and the greatest width is at the intersection of the posterior margin of the anterior depression with the lateral margin ( Fig. 2 View FIGURE 2 A). Whereas, F. d a i d a i sp. nov. and F. tholia have following differences. The ratio of width to length of the anterior carapace depression also varies between species: F. daidai (mean of depression width/length ± SD 1.52 ± 0.14, N = 17); F. h e im i (1.88 ± 0.27, N = 6); F. i s h i k a w a i (1.98, N = 1); F. latisella (2.05 ± 0.12, N = 5); F. stimpsoni (1.68 ± 0.21, N = 11) and F. tholia (1.5, N = 1, from Kropp, 1994) ( Fig. 9 View FIGURE 9 ).
In F. daidai View in CoL sp. nov., the ocular peduncles are mostly exposed in dorsal view ( Figs. 2 View FIGURE 2 A, 4, 7), and the midline of the depression is almost invisible in lateral view ( Fig. 2 View FIGURE 2 B); whereas in F. stimpsoni View in CoL , F. heimi View in CoL , F. latisella View in CoL and F. ishikawai View in CoL , the ocular peduncles are almost hidden under the carapace in dorsal view, with only the corneas visible, and the midline of the depression is visible in lateral view ( Takeda & Tamura 1980, Kropp 1994). The ocular peduncles are mostly hidden in dorsal view in F. tholia View in CoL , and the midline of the depression is visible in lateral view ( Kropp 1994).
The degree of division of anterior carapace depression has been used as species comparison tool.?According to previous studies, in F. h e im i and F. stimpsoni View in CoL , a median longitudinal ridge armed with spines completely divides into two concavities ( Fize & Serène 1957, Takeda & Tamura 1980, Kropp 1994). By contrast, the anterior carapace depressions are incompletely divided in F. latisella View in CoL , F. ishikawai View in CoL and F. tholia View in CoL ( Takeda & Tamura 1980, Kropp 1994). As noted previously, however, division of anterior carapace depression vary by degree at least in F. daidai View in CoL sp. nov. ( Figs. 2 View FIGURE 2 A, 4, 7, 8B). This character is useful for some species ( F. stimpsoni View in CoL for instance) as diagnosis, whereas, unsuitable character for separating Fizesereneia View in CoL species.
Fize & Serène (1957) stressed two features as useful for distinguishing genera in the Cryptochiridae View in CoL : coral host specificity and the degree of exopod development of PLP-2 (PLP on the second somite) in females. Fizesereneia View in CoL species, as far as has been observed, are all found in pits on the surface of massive corals of Indo-Pacific species of the family Mussidae Ortmann, 1890 View in CoL (= the proposed family Echinophylliidae Dai & Horng, 2009, Lobophylliidae Dai & Horng, 2009 View in CoL , or Lobophylliidae Fukami, Budd & Knowlton, 2012 View in CoL in Budd et al., 2012, validity of them still under debate). McCain & Coles (1979), however, emphasized that a generic classification must be based on morphological characters alone, rather than host of coral species. They also suggested that the degree of exopod development in the female PLP-2 cannot accurately reflect phylogenetic relationships among the genera, because that character is highly variable in Utinomiella dimorpha (Henderson, 1906) View in CoL . The PLP-2 of Fizesereneia View in CoL species is known to have a rudimentary exopod in five of the six species (unknown in F. t h o l i a). The biramous PLP-2 was considered one of a shared character among the species in genus Fizesereneia View in CoL . More extensive studies of family Cryptochiridae View in CoL are needed to assess the efficacy of this character as diagnostic character for higher classification. Kropp & Manning (1987) and Kropp (1990) suggested that the pterygostomial region, being either fused or not fused to the carapace, is useful for diagnosing Cryptochirid genera. The pterygostomial region is completely fused to the carapace in Fungicola Serène, 1966 View in CoL , and Utinomiella Kropp & Takeda, 1988 View in CoL , but separated by a soft membrane in Pseudocryptochirus Hiro, 1938 View in CoL , and Xynomaia Kropp, 1990 View in CoL . In the case of Fizesereneia View in CoL , however, our observations show that this character is not consistent across all species in the genus. In F. h e i m i, F. latisella View in CoL , F. ishikawai View in CoL and F. tholia View in CoL , the pterygostomial region is separated by a narrow membrane, but in F. stimpsoni View in CoL and F. daidai View in CoL the pterygostomial region is completely fused to the carapace. Further studies are needed to evaluate the phylogenetic usefulness of this character in other genera.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Fizesereneia daidai Zayasu
| Zayasu, Yuna, Nomura, Keiichi, Seno, Koutaro & Asakura, Akira 2013 |
Fizesereneia latisella
| Kropp 1994: 1 |