Merostenus Walker
|
publication ID |
https://doi.org/10.5281/zenodo.556479 |
|
publication LSID |
lsid:zoobank.org:pub:3EF97DA3-EF37-4D91-8689-2AB9151D1440 |
|
DOI |
https://doi.org/10.5281/zenodo.6049185 |
|
persistent identifier |
https://treatment.plazi.org/id/03C187CB-2269-CF31-FF78-FF0CFAE14C62 |
|
treatment provided by |
Plazi |
|
scientific name |
Merostenus Walker |
| status |
|
Merostenus Walker View in CoL View at ENA
Merostenus Walker, 1837: 354 View in CoL –355. Type species: Merostenus phedyma Walker View in CoL (= Eupelmus excavatus Dalman, 1820 View in CoL ), by monotypy.
Urocryptus Westwood, 1839: 72 . Type species: Eupelmus excavatus Dalman, 1820: 382 View in CoL –383, by monotypy and original designation. Preoccupied by Urocryptus Temminck, 1838: 31 , discovered by Dalla Torre, 1897: 85. Synonymy by Ruschka, 1921: 309.
Eupelminus Dalla Torre, 1897: 85 . Replacement name for Urocryptus Westwood. Synonymy by Ruschka, 1921: 309.
Finlayia Girault, 1934: 1 . Type species: Finlayia puella Girault , by monotypy. Preoccupied by Finlayia Giles, 1904: 365 , discovered by Bouček, 1988: 558. n. syn.
Reikosiella Yoshimoto, 1969: 627 View in CoL –628. Type species: Reikosiella melina Yoshimoto View in CoL , by monotypy and original designation. n. syn.
Hirticauda Bouček, 1988: 558 . Type species: Cerambycobius pax Girault , by original designation. Synonymy under Reikosiella View in CoL by Gibson 1995: 259. n. syn.
Reikosiella ( Capreocauda) Gibson, 1995: 262 . Type species: Idoleupelmus tsaratananae Risbec , by monotypy and original designation. n. syn.
Reikosiella ( Incohata) Gibson, 1995: 263 . Type species: Reikosiella (Incohata) guttata Gibson , by monotypy and original designation. n. syn.
Reikosiella ( Reikosiella) View in CoL ; Gibson, 1995: 265.
Reikosiella ( Hirticauda) View in CoL ; Gibson, 1995: 263.
Diagnosis. FEMALE. Head with scrobal depression subcircular to broadly oval, the lateral margin often directed obliquely from torulus towards and sometimes to inner orbit, but lateral and dorsal margins not carinately margined. Mandibles tridentate. Antenna sometimes bicolored with scape, pedicel or one or more funiculars pale to white; with one anellus (fl1). Maxillary palpus normal, not unusually long. Pronotal collar divided medially or ( excavatus species-group) completely sclerotized. Structures of dorsellum and propodeum relative to apex of scutellum highly variable (characteristic of subgenus). Wings fully developed or strongly reduced; macropterous individuals without linea calva and with marginal vein comparatively long, distinctly longer than costal cell; brachypterous individuals, except for M. melinus , with fore wing extending at most to about level of posterior margin of Gt1 if held flat over body and hyaline to variably infuscate without transverse band or anterior and posterior hyaline spots with white setae. Middle leg without groove apically between mesotibial spur and tarsus and with or without apical pegs over base of mesotarsus (depending on subgenus) but pegs, when present, of similar color as tibia and sometimes difficult to discern; mesotarsus with pegs of similar color as tarsus and with single row of pegs along either side of basitarsus. Metatibia not conspicuously compressed. Prepectus with frontal surface small and not contrasting in color with surrounding cuticle. Acropleuron bare posterior to setose mesopectal region below prepectus; variable in structure posteriorly (characteristic of subgenus). Gaster with penultimate tergite exposed anterior to syntergum, not divided mediolongitudinally, and usually more or less transverse-rectangular rather than tapered posteriorly; syntergum, except rarely, with dorsal surface extensive anterior to posterior margin, but structure variable posteriorly, most often deeply emarginate (omega-like: Ω) but sometimes posterior margin more or less transverse in dorsal view; anal tube with variably melanized subanal plate ventral to anus and, except for R. ( Incohata ), almost always with distinct, though sometimes yellowish supra-anal plate dorsal to anus contiguous with posterior margin of syntergum. Ovipositor sheaths variable, though often long and filamentous when syntergum omega-like emarginate and short and rigid when syntergum not distinctly emarginate.
MALE. Diagnosis as given by Gibson (1995) for Reikosiella except for following changes or additions: head—vertex sometimes with variably distinct sulcus or line between anterior margin of posterior ocellus and inner orbit; flagellum—funiculars obviously longer than wide with variably long and dense mps and comparatively long but variably dense setae, but at least mps not in multiple rows per funicular and without long, apically free, setalike portion, and setae at least about as long as width of funicular even if comparatively sparse and subapressed to funicular rather than more conspicuously projecting out from funicular; mesoscutum—variably sculptured, but usually meshlike coriaceous to coriaceous-imbricate (at least New World males); metapleuron—bare or partly setose; fore wing—disc dorsally entirely setose or sometimes bare behind parastigma and base of marginal vein to basal and mediocubital folds, but bare region not conspicuous because ventral surface setose, or very rarely with oblique bare band similar to linea calva separated by setae from venation and discal folds; metasoma—gaster entirely dark or partly paler basally to subbasally.
Distribution. Based on examined specimens, all four recognized subgenera— M. ( Capreocauda ), M. ( Incohata ), M. ( Merostenus ) and M. ( Reikosiella ) occur in the New World, both Nearctic and Neotropical, even though species of M. ( Capreocauda ) and M. ( Merostenus ) have yet to be recorded from these regions (see species checklist). Species of M. ( Capreocauda ) occur also in the Afrotropical, Australasian and Oriental regions, but I have yet to see specimens from the Palaearctic. Species of M. ( Incohata ) and M. ( Reikosiella ) occur only in the New World, other than M. ( Reikosiella) melinus , which likely was introduced to Hawaii from South America (see species treatment). Though relatively few species are yet described, the New World fauna of Merostenus is dominated by species of M. ( Reikosiella ), whereas M. ( Merostenus ) is most diverse in the Old World where it occurs in all biogeographic realms.
Biology. Of 51 described species of Merostenus , the only one for which hosts and biology are confidently known through multiple rearings is M. ( Merostenus) excavatus , which is an egg predator of species of Hypera Gemar ( Coleoptera : Curculionidae ), including the alfalfa weevil and clover leaf weevil ( Chamberlin 1924b). Larvae estivate in the stems of alfalfa prior to adults emerging ( Chamberlin 1924b). Fusu (2013) cited galls of Cynipidae (Hymenoptera) as rearing records for three other species of M. ( Merostenus ) from Europe— M. bolivari (Kalina) n. comb., M. hungaricus (Erdős) n. comb., and M. rostratus (Ruschka) n. comb. However, he noted that none of these records prove cynipids as the actual hosts for the three species. Although individuals of M. rostratus have been reared several times from cynipid galls, Bouček (1977) thought that eggs of Orthoptera deposited on the galls were likely the true hosts. Fusu (2013) also suggested that two other rearing records for M. rostratus could indicate it as a parasitoid of inquiline caterpillars in cynipid galls on oaks or some other Lepidoptera larvae associated with oak trees that pupate in hollow galls or under the bark. Single individuals of M. bolivari were also reared from cynipid galls in France and Spain, but subsequent attempts to rear more specimens in Spain failed and it was suggested the specimens may have emerged from hidden eggs or from some other host in empty galls ( Fusu 2013).
In describing M. ( Reikosiella) melinus, Yoshimoto (1969) stated that this species was reared from the larvae or pupae of Alucita objurgatella (Walsingham) ( Lepidoptera , Alucitidae ), though this record also requires confirmation (see under species treatment). The CNC has four females representing two undescribed species of M. ( Reikosiella ) from USA reared from cynipid galls—two from a single rearing in Arizona from galls of Dryocosmus coxii (Bassett) on Quercus emoryi Torr. (Fagaceae), and two from a single rearing in Georgia labelled with “Chestnut gall” (likely a gall on Castanea dentata (Marsh) Borkh. ( Fagaceae )). These are the only host records among numerous specimens of M. ( Reikosiella ) in the CNC from throughout North America. Finally , based on label data, Gibson (1995) reported Grapholita packardi (Zeller) ( Lepidoptera : Tortricidae ) as a host for an unidentified species of M. ( Capreocauda ) from British Columbia , Canada. He also stated he saw one specimen labelled as “ex. gall” ( Georgia, USA) and another as bred from Vachellia farnesiana (L.) Wight & Arn. ( Fabaceae ) ( Texas, USA) . The CNC has another female labelled from British Columbia as associated with Douglas fir, Pseudotsuga menziesii (Mirb.) Franco (Pinaceae) .
Far too few verified host records are known to confidently predict either the host range or biology of species of Merostenus . Although several records indicate an association of species of M. ( Merostenus ), M. ( Reikosiella ) and M. ( Capreocauda ) with cynipid galls, the very few reared specimens relative to the number of times cynipid galls have been reared over the years suggests these might be fortuitous associations and Cynipidae are not the actual hosts. Fusu (2013) further suggested that, based on detailed collection records, species of M. ( Merostenus ) have arboreal lifestyles. Although this appears to apply to most Merostenus species where biological information is known, it does not for M. excavatus , which has brachypterous females. Additional research is necessary to determine whether there are any host or other biological differences between species having macropterous or brachypterous females.
Brachyptery. Female brachyptery in Merostenus is known only for M. ( Reikosiella) melinus and several species of M. ( Merostenus ), mostly from the Afrotropical region. Polymorphism in wing length is known only for M. melinus , females being macropterous to variably strongly brachypterous ( Yoshimoto 1969). Brachypterous females of M. ( Merostenus ) sometimes have the fore wings slightly infuscate, but only those of M. melinus typically have a hyaline cross-band with white setae behind the marginal vein apically, similar to macropterous females of the species. This latter color pattern is similar to that of many species of Anastatus Motschulsky with either macropterous or brachypterous females.
Generic limits and relationships. Couplet one of Gibson’s (1995) key to genera of Eupelminae divided females into two groups based on three features—structure of the syntergum, presence or absence of a mesotibial apical groove and, when present, relative position of the mesotibial apical pegs. Keyed through the first half of the couplet were females with an apically deeply emarginate syntergum ( Gibson 1995, character 39, state 2; figs 311– 313, 315–324) in combination with a mesotibia without a apical groove ( Gibson 1995, character 34, state 1; figs 331–342) and either without apical pegs ( Gibson 1995, character 35, state 1; figs 337, 338) or, when present, with these positioned at least partly over the base of the tarsus ( Gibson 1995, character 35, state 2; figs 331–336, 339– 342). Keyed through the second half of couplet one were females with an apically truncate (character 39, state 1) or posteriorly rounded (character 39, state 3) syntergum and/ or a mesotibia with an apical groove ( Gibson 1995, character 34, state 2; figs 327–330) and with apical pegs restricted to a narrow region above the base of the mesotibial spur ( Gibson 1995, character 35, state 3; figs 327–330). An apically truncate syntergum, absence of a mesotibial apical groove, and absence of mesotibial apical pegs were all hypothesized as the groundplan states of the respective characters for female Eupelminae . Based on the different character-state combinations, Reikosiella and five other genera ( Australoodera Girault , Ecnomocephala Gibson , Eupelmus Dalman , Phlebopenes Perty and Tineobius Ashmead ) were keyed through the first half of couplet one, whereas Merostenus was keyed through the second half of the couplet along with all other eupelmine genera. This included Omeganastatus Gibson and Brasema Cameron , even though females of the former genus and some females of the latter genus have a deeply emarginate syntergum ( Gibson 1995, fig. 314), because their mesotibia has an apical groove and apical pegs over the base of the tibial spur. Females of the two genera also share what was hypothesized as the apomorphic structure of the mesotrochantinal plate, i.e. consisting of a narrow, flat to slightly convex plate terminated in tiny articulatory lobes ( Gibson 1995, character 22, state 2; fig. 94). Because of the character-state combination, a deeply emarginate syntergum was hypothesized to have evolved in Omeganastatus and in a few species of Brasema independently from those genera keyed through the first half of the couplet, likely in association with secondarily lengthened, filamentous ovipositor sheaths ( Gibson 1995).
Although no evidence was presented that they represent a monophyletic group, Gibson (1995, figs 515, 516) illustrated a group of 10 genera as constituting a mostly unresolved basal group within Eupelminae based on retention of the hypothesized symplesiomorphic structure of the mesotrochantinal plate, i.e. consisting of two partly to completely separated articulatory lobes ( Gibson 1995, character 22, state 1; fig. 93). Included in the group were the six genera listed above that were keyed through the first half of couplet one plus Merostenus , Mesocomys Cameron , Phenaceupelmus Gibson and Xenanastatus Bouček ( Gibson 1995, table 1). Although structure of the mesotrochantinal plate is variable in Reikosiella , it was included because females of the hypothesized most basal subgenus, R. ( Incohata ), possess the plesiomorphic structure, as do at least some species of the other three other subgenera that were classified in the genus. Furthermore, females of Reikosiella lack a mesotibial apical groove ( Gibson 1995, fig. 342), which is shared with the other nine genera having a plesiomorphic structure of the mesotrochantinal plate.
Gibson (1989) hypothesized Eupelminae as a monophyletic group based primarily on two features, extreme sexual dimorphism (character 1, state 2), which is a composite of several features, and, for females, reduction of the t2–tr2 (mesotergal-mesotrochantinal) muscle from a large tubular muscle originating from each axilla and axillar phragma ( Gibson 1989, character 17, state 1; fig. 131) to a slender, tendon-like muscle originating from the apex of the ventroapical angle of the lateral surface of the axilla ( Gibson 1989, character 17, state 6; fig. 132). Subsequently, Gibson (1995, figs 515, 516) hypothesized the monotypic genus Phenaceupelmus Gibson as the basal-most lineage of Eupelminae based on several external features, including possession of the hypothesized plesiomorphic structures of the mesotrochantinal plate and syntergum for females. This basal relationship was later supported by the discovery that females as well as males of P. chilensis Gibson possess the symplesiomorphic structure of the t2-tr2 muscle for Eupelmidae (Heraty et al. 2013) . Gibson’s (1989) original hypothesis of reduction of the t2-tr2 muscle in females as a synapomorphy for Eupelminae was based on dissections of females of only three genera, one with the plesiomorphic mesotrochantinal plate structure ( Eupelmus ) and two with the apomorphic structure ( Anastatus and Brasema ). Because of the discovery that P. chilensis females possess the plesiomorphic t2-tr2 structure, females of additional genera, as listed under ‘Material and methods’, were dissected for the present study. This included those of all the genera listed above as comprising a basal group possessing separate mesotrochantinal lobes plus four additional genera possessing the apomorphic mesotrochantinal lobe structure. All the newly dissected females were found to have similar tendon-like structures of t2-tr2 originating from the apex of the ventroapical angle of the lateral surface of the axilla. This more comprehensive character-state survey therefore more strongly supports Phenaceupelmus as the sister group of remaining Eupelminae . The basal position of Phenaceupelmus within Eupelminae was important for the phylogenetic analysis of Gibson (1995) because most character-state polarities were based on the hypothesis that individuals of P. chilensis retain the symplesiomorphic groundplan structures of the subfamily.
In addition to mesotrochantinal lobe structure, females of P. chilensis were recorded as also having the hypothesized plesiomorphic structure of the syntergum, i.e. a dorsally flat to evenly convex, apically undifferentiated tergite having a more or less transverse posterior margin ( Gibson 1995, character 39, state 1; figs 271–278). Two other syntergal structures were described as apomorphic states, the apically omega-like emarginate syntergum, and one in which the syntergum is tapered and constricted posteriorly into a narrow, posteriorly rounded or angulate margin and/or reflexed into a rim or posteriorly rounded flange ( Gibson 1995, character 39, state 3; figs 283–306). Both the emarginate and flanged syntergal structures were hypothesized to have evolved independently from a plesiomorphic structure similar to that possessed by P. chilensis ( Fig. 1 View FIGURES 1 – 8 ). The plesiomorphic syntergal structure was also recorded for R. ( Incohata ) ( Gibson 1995, figs 309, 310), the hypothesized basal-most lineage of Reikosiella . Its basal position within Reikosiella was postulated primarily because of relative structure of the scutellum, dorsellum and propodeum (sdp-complex) ( Gibson 1995, character 24, state 1; fig. 189), which is also very similar to that of P. chilensis ( Gibson 1995, fig. 187). Because of the combination of sdp-complex and syntergal structure for R. ( Incohata ) it was further suggested that the deeply emarginate synterga characteristic of almost all other Reikosiella ( Gibson 1995, table 1; figs 311–313) evolved convergently to similar deeply emarginate synterga that characterize females of Australoodera ( Gibson 1995, fig. 318), Ecnomocephala ( Gibson 1995, fig. 319), Eupelmus ( Gibson 1995, figs 320–324), Phlebopenes ( Gibson 1995, fig. 316) and Tineobius ( Gibson 1995, fig. 317). Females of R. ( Capreocauda ), R. ( Hirticauda ) and R. ( Reikosiella ) were recorded as having different, variably derived structures of the sdp-complex, as were females of the above five genera except for some Australoodera ( Gibson 1995, table 1).
In addition to P. chilensis and R. ( Incohata ), females of Merostenus were also recorded as having the plesiomorphic structure of the syntergum, except for one species here described as M. mexicanus , which was stated as having the syntergum reflexed apically into a posteriorly rounded syntergal flange ( Figs 75, 81 View FIGURES 74 – 82 ). Females of Mesocomys ( Gibson 1995, fig. 300) and Xenanastatus ( Gibson 1995, fig. 306) also have variably developed syntergal flanges, as do females of most genera with the apomorphic structure of the mesotrochantinal plate ( Gibson 1995, table 1). Structure of the sdp-complex was not coded for genera composed only of species with brachypterous females, such as Merostenus , because Gibson (1995) noted that the sdp-complex tends to be highly modified for brachypterous females in genera with both macropterous and brachypterous females. However, the sdp-complex structure of females that would be assigned to Merostenus is more similar to that of R. ( Hirticauda ) than the other subgenera of Reikosiella because the dorsellum abuts the apex of the scutellum so that in lateral view the scutellum is not protuberant, unlike females of R. ( Reikosiella ) ( Gibson 1995, fig. 176) and R. ( Capreocauda ) ( Gibson 1995, fig. 180), and the propodeum is variably modified from that of R. ( Incohata ) females ( Gibson 1995, fig. 189).
Very few genera other than Phenaceupelmus , R. ( Incohata ) and Merostenus were recorded by Gibson (1995, table 1) as possessing a plesiomorphic syntergal structure, primarily Brasema Cameron and Calymmochilus Masi , though both states 1 (unmodified) and 3 (flanged) or structures difficult to assign unambiguously to either state were recorded for some genera. In reassessing the difference between an unmodified syntergum and one with a syntergal flange for this study, particularly the difference between the syntergal structures typical of Brasema ( Gibson 1995, fig. 272) and Zaischnopsis Ashmead ( Gibson 1995, figs 305, 306), it was realized that the hypothesized symplesiomorphic structure should likely also include a row of elongate setae that originate slightly anterior to the posterior margin of the syntergum and extend conspicuously over the margin (e.g. Figs 1 View FIGURES 1 – 8 , 19 View FIGURES 15 – 21. 15 – 18 ). Females of P. chilensis have such a row of setae ( Fig. 1 View FIGURES 1 – 8 ), as do most species of Brasema ( Fig. 19 View FIGURES 15 – 21. 15 – 18 ), whereas females with a syntergal flange lack setae paralleling the margin, though often there are more conspicuously premarginal setae whose apices project, at most, just beyond the syntergal apex ( Gibson 1995, figs 284, 286, 290, 292, 296, 298, 305, 306). This setal difference indicates that syntergal flanges evolved through secondary expansion of the sublinear region of cuticle posterior to the line of submarginal setae characteristic of such taxa as P. chilensis ( Fig. 1 View FIGURES 1 – 8 ) and Brasema ( Fig. 19 View FIGURES 15 – 21. 15 – 18 ). The different setal patterns can be used as a supplemental feature to differentiate females of most species of Brasema and Zaischnopsis . However, Gibson (1995) hypothesized that Zaischnopsis might render Brasema paraphyletic ( Gibson 1995, fig. 517), and the syntergal structure/setal patterns of females of the Brasema schizomorpha -group sensu Gibson (1995) could support such a hypothesis. Females of the schizomorpha - group have the posterior margin of the syntergum slightly incurved rather than posteriorly rounded, and the apicalmost setae in a transverse row with their apices projecting somewhat beyond the posterior margin, but the setae are quite distinctly premarginal because there is a transverse, lighter-colored to translucent, flat or slightly reflexed cuticular region posterior to them ( Fig. 19 View FIGURES 15 – 21. 15 – 18 : insert). Either the syntergal structure/setal patterns of schizomorpha - group and Zaischnopsis females evolved independently or the schizomorpha -group represents a clade of species in which the cuticle posterior to the premarginal setae increased prior to the posterior margin becoming posteriorly rounded. If so, schizomorpha -group species are indicated to be more closely related to Zaischnopsis than to other Brasema and therefore are incorrectly classified. Syntergal setation is variable for museum specimens of R. ( Incohata ), but some females have two long premarginal setae paramedially ( Fig. 3 View FIGURES 1 – 8 ) or even more numerous premarginal setae.
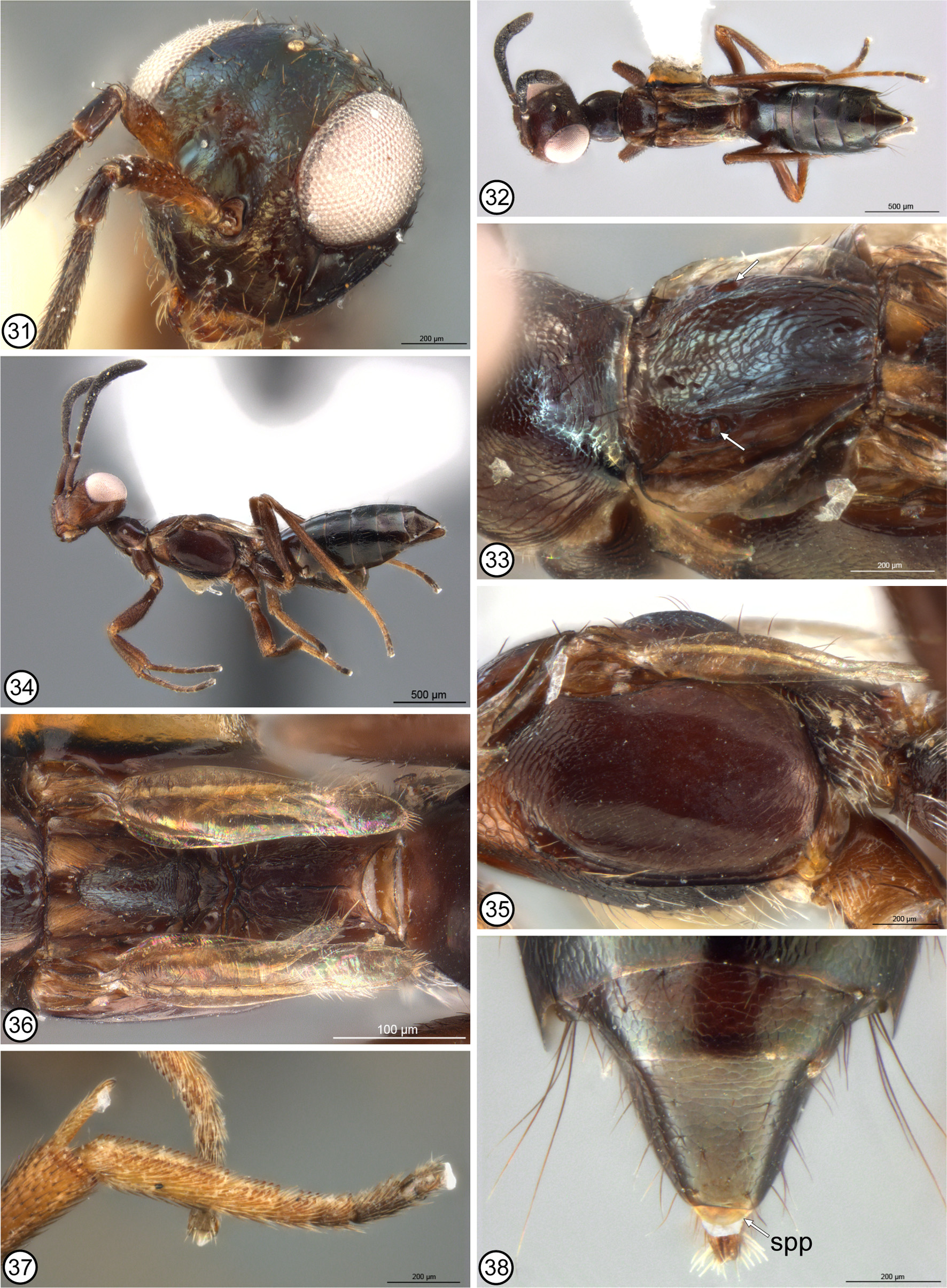
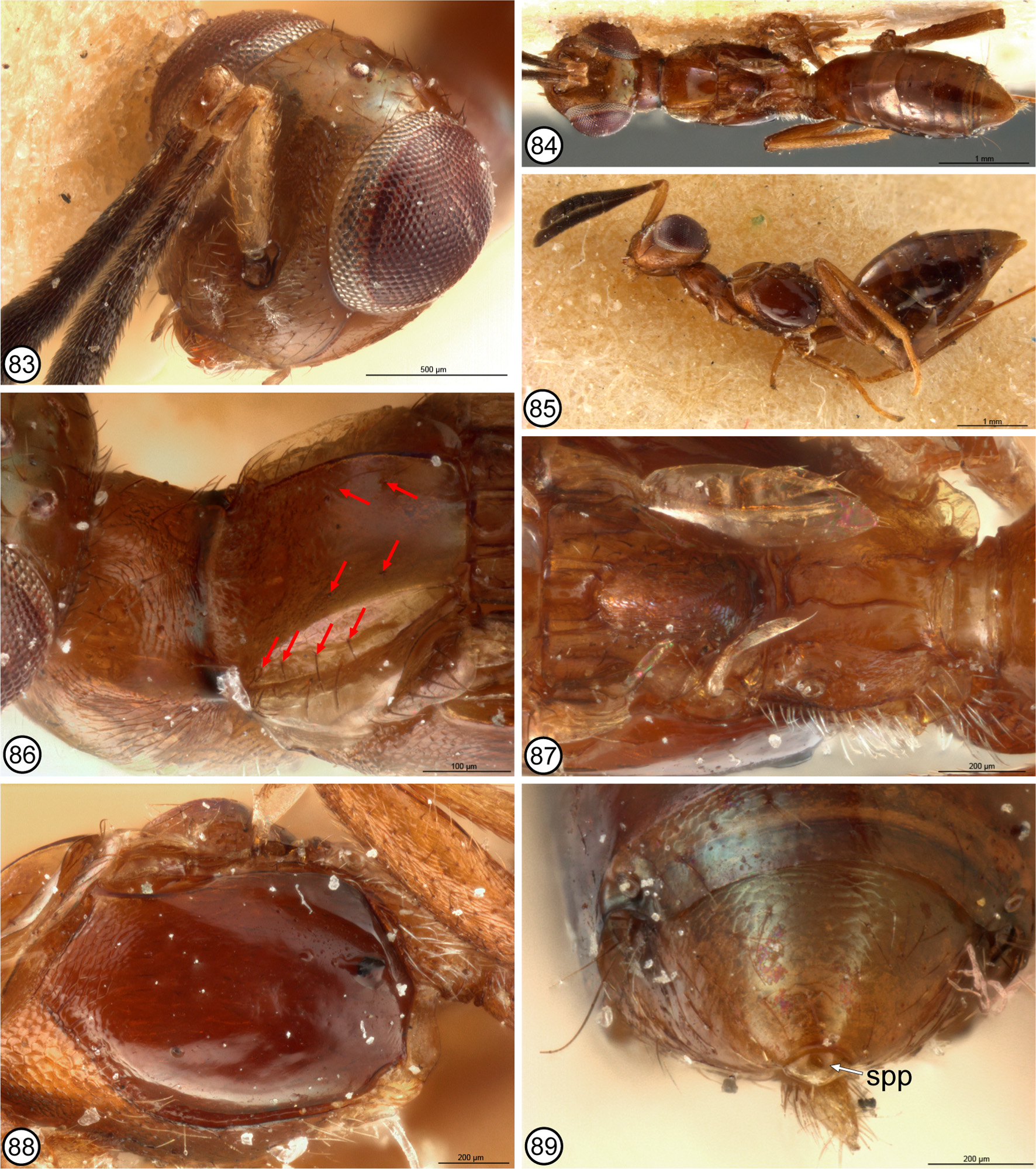
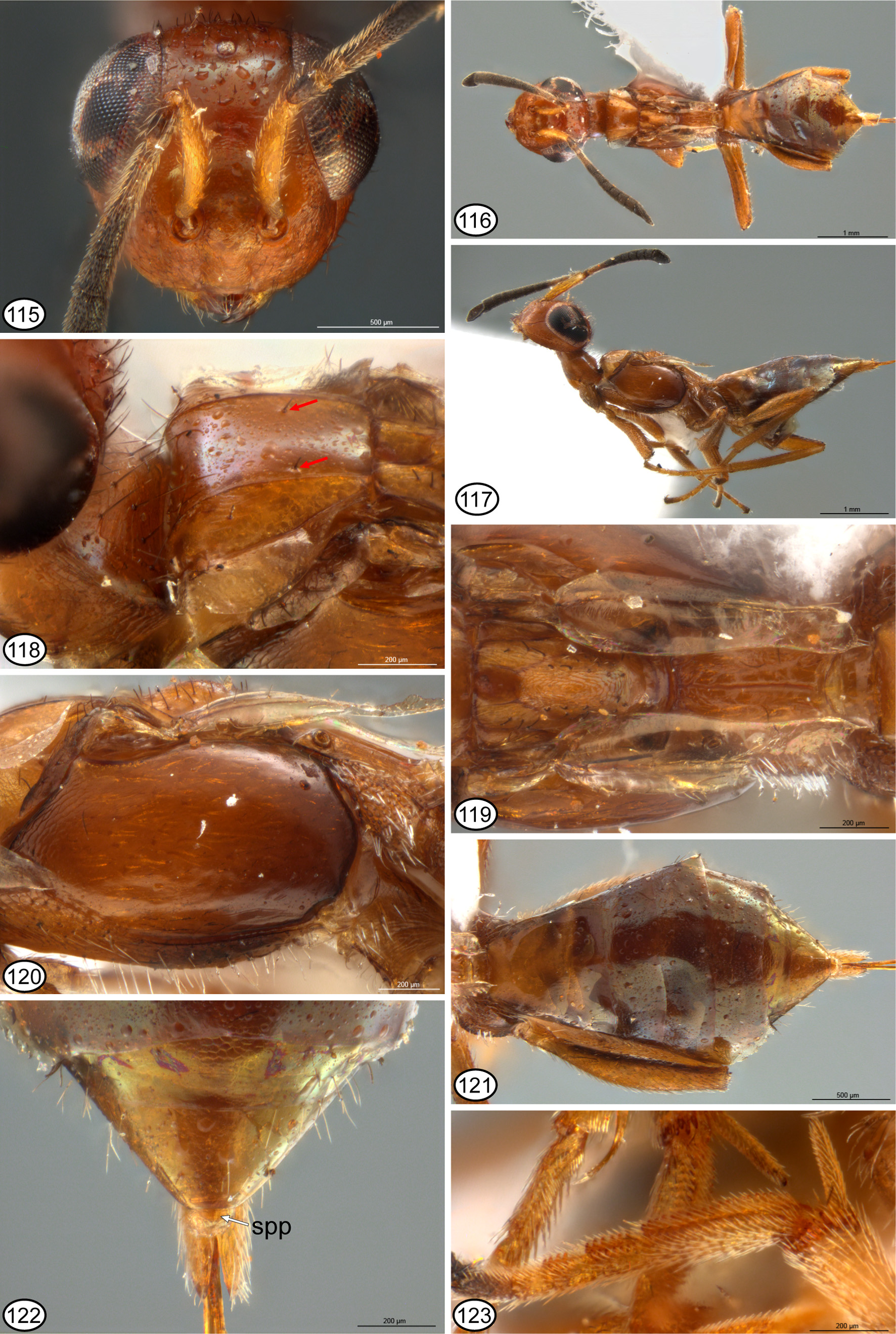
Although not analyzed as a character within Eupelminae , Gibson (1995) also noted the presence of a melanized sclerite, which he called the anal sclerite ( Gibson 1995, figs 320, 323: asc), in some females with a deeply emarginate syntergum. A similar melanized sclerite is usually also visible in ventral view under the apex of an unmodified syntergum or one with a syntergal flange, though sometimes it is concealed between the syntergum and ovipositor sheaths. In all instances this sclerite is separated from the posterior margin of the syntergum by membrane. Gibson (1995, p. 44) hypothesized that the former structure was a consequence of the syntergum being “shortened dorsally [because of development of a deep emargination], resulting in the primitively concealed anal sclerite being pulled from a ventral position to a posteriorly or dorsally directed position”. However, examination of the syntergal structure of females that would be classified in Merostenus sensu Gibson (1995) , and subsequent re-examination of the structures of females of other genera with a deeply emarginate syntergum shows the observation of structure was inaccurate. The single known female of M. mexicanus has a melanized sclerite faced ventrally under the reflexed apex of the syntergum ( Figs 81, 82 View FIGURES 74 – 82 ), and the dorsal surface of the syntergum is sparsely setose with similarly short setae, including along the extreme posterior margin ( Figs 75, 82 View FIGURES 74 – 82 ). However, except for M. platyscapus , at least some females of the other species here classified as the excavatus group of M. ( Merostenus ) (= Merostenus sensu Gibson 1995 ), have two externally visible syntergal sclerites. There is a variably transverse sclerite faced dorsally or posteriorly behind and contiguous with the posterior margin of the syntergum ( Figs 38 View FIGURES 31 – 38 , 47, 48 View FIGURES 39 – 48 , 89 View FIGURES 83 – 89 , 105 View FIGURES 98 – 105 ) and a ventral, suboval sclerite that is separated from the dorsal sclerite by membrane, which sometimes is extended posteriorly into an ‘anal tube’ ( Figs 14 View FIGURES 9 – 14 , 16 View FIGURES 15 – 21. 15 – 18 : ant). When an anal tube is evident, more typically in critical-point dried than air-dried females, the dorsal sclerite is seen to lie dorsal to and the ventral sclerite ventral to the terminal anus ( Figs 14 View FIGURES 9 – 14 , 16–18 View FIGURES 15 – 21. 15 – 18 : an). Consequently, the more dorsal sclerite is here called the ‘supra-anal plate’ ( Figs 38 View FIGURES 31 – 38 , 47, 48 View FIGURES 39 – 48 , 89 View FIGURES 83 – 89 , 105 View FIGURES 98 – 105 , 122 View FIGURES 115 – 123 : spp) and the ventral sclerite the ‘subanal plate’ ( Figs 48 View FIGURES 39 – 48 , 81, 82 View FIGURES 74 – 82 : sbp), to reflect the double structure and position of each relative to the anus (see further below). Females of M. excavatus are variable in development of the supra-anal plate. Some females have quite a distinct, dark supra-anal plate whereas others just a slightly yellowish, strongly transverse, inconspicuous supra-anal plate that abuts the posterior margin of the syntergum ( Fig. 47 View FIGURES 39 – 48 ) or is more conspicuous behind the syntergum if the anal tube is extended ( Fig. 48 View FIGURES 39 – 48 ). Some females also appear to lack a supra-anal plate, but based on presence in many females this likely is because in air-dried females the anal tube typically is collapsed within the gaster and the supra-anal plate is then sometimes hidden under the apex of the syntergum. Known females of M. platyscapus also lack a visible supra-anal plate, but true absence requires confirmation through observation of freshly collected specimens because apparent absence in available specimens might result from the sclerite being hidden under the syntergum apically. Setation of the syntergum of excavatus -group females is variable, often being bare dorsally, but if setose then evenly setose without a line of longer premarginal setae that project distinctly beyond the posterior margin. Females of P. chilensis that have the anal tube extended ( Fig. 1 View FIGURES 1 – 8 : ant) lack an evident supra-anal plate and in ventral view the subanal plate is very slender, being strongly melanized/sclerotized only mediolongitudinally ( Fig. 2 View FIGURES 1 – 8 : sbp). Within what was classified as Reikosiella sensu Gibson (1995) , females of R. ( Incohata ) also lack a distinct supraanal plate, though sometimes the membrane above the anus is yellowish, being slightly sclerotized/melanized ( Fig. 3 View FIGURES 1 – 8 : spp), in critical-point dried females having the anal tube extended posteriorly ( Fig. 3 View FIGURES 1 – 8 : ant). Females of R. ( Incohata ) also have a variably strongly but more extensively sclerotized/melanized subanal plate below the anus than for P. chilensis , being more oval ( Fig. 4 View FIGURES 1 – 8 ) similar to the subanal plate characteristic of females of the other three subgenera ( Figs 5–7 View FIGURES 1 – 8 ) and other female Eupelminae ( Figs 12–14 View FIGURES 9 – 14 , 16 View FIGURES 15 – 21. 15 – 18 ). A revision of the species of the subgenera is required in order to document character-state distribution accurately, but at least the vast majority of females of R. ( Capreocauda ) ( Fig. 5 View FIGURES 1 – 8 ), R. ( Hirticauda ) ( Figs 6 View FIGURES 1 – 8 , 10 View FIGURES 9 – 14 ) and R. ( Reikosiella ) ( Figs 7–9 View FIGURES 1 – 8 View FIGURES 9 – 14 ) sensu Gibson (1995) have a distinctly melanized, variably large supra-anal plate adjacent to the syntergal emargination in addition to a variably melanized, suboval subanal plate ventrally. The presence of such a strongly sclerotized/melanized supra-anal plate that it appears as a separate sclerite along the posteromedial margin of the syntergum is undoubtedly a derived feature. Further, the vast majority of females of the three subgenera lack or have only a couple of long syntergal premarginal setae paramedially ( Figs 5–7 View FIGURES 1 – 8 , 9, 10 View FIGURES 9 – 14 ), though rarely there is a complete row ( Fig. 8 View FIGURES 1 – 8 ). This indicates that syntergal premarginal setae were secondarily lost within Reikosiella , but more accurate knowledge of characterstate distribution is required to determine whether a complete row was the groundplan state for the genus and, if so, how many times loss to two paramedial or no premarginal setae occurred. Of the other basal genera with a distinctly emarginate syntergum, females of Australoodera ( Fig. 11 View FIGURES 9 – 14 ), Ecnomocephala ( Fig. 13 View FIGURES 9 – 14 ) and Phlebopenes ( Fig. 14 View FIGURES 9 – 14 ) are similar to those of R. ( Incohata ) in at least lacking a strongly melanized/sclerotized supra-anal plate, though when the anal tube is extended posteriorly the dorsal membrane anterior to the anus is often slightly melanized/sclerotized ( Figs 11, 14 View FIGURES 9 – 14 ). There is also a similarly or more strongly melanized subanal plate ( Figs 11, 13, 14 View FIGURES 9 – 14 ). Females of Australoodera ( Fig. 11 View FIGURES 9 – 14 ; Gibson 1995, fig. 318) and Ecnomocephala ( Fig. 13 View FIGURES 9 – 14 ; Gibson 1995, fig. 319) have a very short, sublinear dorsal surface of the syntergum ( Gibson 1995, table 1, character 39, state 2b) and at most two long premarginal setae paramedially ( Fig. 11 View FIGURES 9 – 14 ). Females of Phlebopenes have an extensive dorsal surface of the syntergum anterior to the emargination ( Gibson 1995, table 1, character 39, state 2a) and numerous premarginal setae that project conspicuously beyond the syntergal emargination ( Fig. 14 View FIGURES 9 – 14 ; Gibson 1995, fig. 316).
Most examined museum females of T. ( Tineobius ) have the syntergal emargination partly to completely hidden under one or more preceding tergites, but the dorsal surface of the syntergum anterior to the emargination is sublinear ( Fig. 12 View FIGURES 9 – 14 ; Gibson 1995, fig. 317). Syntergal premarginal setae were not observed for any female in which the slender dorsal surface was visible, but a subanal plate and variably melanized supra-anal plate are present. Females of some species have a comparatively lightly sclerotized, yellowish supra-anal plate similar to the preceding genera, but at least some have a more distinctly melanized supra-anal plate ( Fig. 12 View FIGURES 9 – 14 ) similar to Reikosiella sensu Gibson (1995) excluding R. ( Incohata ). Finally, females of most species of Eupelmus have a deep syntergal emargination partly to completely surrounding a single visible melanized plate ( Gibson 1995, figs 320– 324), but whether this is the supra-anal or subanal plate differs among species. The single, typically strongly sclerotized/melanized plate of E. ( Eupelmus ) and E. ( Macroneura ) females ( Gibson 1995, figs 323, 324) is the subanal plate based on its position ventral to the anus ( Fig. 16 View FIGURES 15 – 21. 15 – 18 : insert) and often the presence of a more lightly melanized/sclerotized supra-anal plate dorsal to the anus when the anal tube is extended ( Fig. 16 View FIGURES 15 – 21. 15 – 18 ). The latter plate is normally concealed within the gaster when the anal tube is not extended. In the hypothesized basal grade of Eupelmus , E. ( Episolindelia Girault ) ( Gibson 1995, figs 321, 322), females of what Gibson (1995) differentiated as the australiensis group, those with the gaster obviously tapered posteriorly and with a terminal syntergal emargination, either have both a supra-anal and subanal plate externally visible within the emargination ( Fig. 15 View FIGURES 15 – 21. 15 – 18 ; Gibson 1995, fig. 322: note transverse line that separates the two sclerites behind the syntergal premarginal setae) or just the supra-anal plate visible when the anal tube is not extended. The two plates, particularly the subanal plate, are often only lightly melanized/sclerotized so that presence or absence of an external subanal plate is often difficult to determine confidently, but when both are obvious the supra-anal plate is anterior to the subanal plate ( Fig. 15 View FIGURES 15 – 21. 15 – 18 ) similar to some Reikosiella with a similar apical structure of the syntergum ( Fig. 5 View FIGURES 1 – 8 ). Gibson (1995) also differentiated the hartigi -group within E. ( Episolindelia ). Females of this group often have the gaster flattened apically, but at least the syntergal emargination is faced more dorsally and the syntergum extends posterior of the emargination to some extent. Such females also have only a single, typically more strongly sclerotized/melanized plate visible when the anal tube is not extended ( Gibson 1995, fig. 320: asc). However, this sclerite is seen to be the supra-anal plate if the anal tube is extended ( Figs 17, 18 View FIGURES 15 – 21. 15 – 18 ). Therefore, the sclerites labelled as “asc” (anal sclerite) in Gibson (1995, figs 320, 323) are not the same sclerite but the supra-anal sclerite in fig. 320 and the subanal sclerite in fig. 323. The difference in structure between the hartigi -group of E. ( Episolindelia ) and that of E. ( Eupelmus ) and E. ( Macroneura ) is clearly evident only if the anal tube is extended. Gibson (1995) stated that females of E. memnonius Dalman , the type species of Eupelmus , are structurally intermediate between those of the E. ( Episolindelia) australiensis -group and those of E. ( Eupelmus ), and only questionably classified E. memnonius in E. ( Eupelmus ) based on the presence of dark mesotarsal pegs that are slightly differentiated into two rows apically on the basitarsus. An image of the syntergum of E. memnonius ( Fig. 15 View FIGURES 15 – 21. 15 – 18 : insert) taken by Lucian Fusu (Al. I. Cuza University, Iasi, Romania) shows that structure is most similar to E. ( Episolindelia) australiensis -group females, including the presence of externally visible supra-anal and subanal plates when the anal tube is not extended. This suggests that a terminal, more or less obliquely angled syntergal emargination with externally visible supra-anal and subanal plates are groundplan features for Eupelmus . Presence of just an externally visible supra-anal plate for E. ( Episolindelia) hartigi -group females, and just a subanal plate for E. ( Eupelmus ) + E. ( Macroneura ) females, also indicates these two clades represent independent monophyletic lineages, with E. memnonius possibly the basal clade of E. ( Eupelmus ) + E. ( Macroneura ) based on symplesiomorphic syntergal anal plate structures but a more derived mesotarsal peg structure relative to E. ( Episolindelia ) females. In all instances within Eupelmus there is again a maximum of two syntergal premarginal setae paramedially ( Figs 15, 16 View FIGURES 15 – 21. 15 – 18 ; Gibson 1995, figs 321, 322).
Females of Calymmochilus , which were included in the basal group of Eupelminae based on mesotrochantinal plate structure, lack any evident supra-anal plate behind the transverse posterior margin of the syntergum, suggesting a plesiomorphic structure except for a reduced number of syntergal premarginal setae. Females of most species have the syntergum bare posterodorsally, though I have seen very few females with two long premarginal setae paramedially. Of those genera with the apomorphic structure of the mesotrochantinal plate ( Gibson 1995, figs 519, 520), all apparently also lack a sclerotized/melanized supra-anal plate when the anal tube is extended, including females of Omeganastatus macrocercus Gibson ( Fig. 21 View FIGURES 15 – 21. 15 – 18 ) and those of Brasema , whether the posterior margin is distinctly emarginate ( Fig. 20 View FIGURES 15 – 21. 15 – 18 ) or not ( Fig. 19 View FIGURES 15 – 21. 15 – 18 ). Females of at least the vast majority of Brasema species have a line of syntergal premarginal setae ( Figs 19, 20 View FIGURES 15 – 21. 15 – 18 ), but these are lacking from O. macrocercus females ( Fig. 21 View FIGURES 15 – 21. 15 – 18 ).
Based on the above character analysis, Gibson’s (1995) coding of the groundplan syntergal structure of Brasema as symplesiomorphic is supported. Syntergal structure of Calymmochilus is also similar to the hypothesized plesiomorphic structure except for a reduced number or lack of any premarginal setae, and their syntergal structure could have evolved simply through secondary loss of the premarginal setae. However, even though females of species of Merostenus sensu Gibson (1995) have very similar syntergal structures as for Calymmochilus females, their coding as plesiomorphic by Gibson (1995) is undoubtedly incorrect. This is evidenced by the presence of a comparatively strongly sclerotized/melanized supra-anal plate along the posterior margin of the syntergum in combination with the lack of a differentiated line of syntergal premarginal setae for M. excavatus ( Figs 47, 48 View FIGURES 39 – 48 ). Of six other newly described species that would have been classified in Merostenus based on the combination of female brachyptery, completely sclerotized pronotum, and apically transverse syntergum, four have similar syntergal structures and setal patterns ( Figs 38 View FIGURES 31 – 38 , 89 View FIGURES 83 – 89 , 105 View FIGURES 98 – 105 , 122 View FIGURES 115 – 123 ) as for M. excavatus . Available females of M. mexicanus ( Fig. 82 View FIGURES 74 – 82 ) and M. platyscapus ( Fig. 96 View FIGURES 90 – 97 ) lack visible supra-anal sclerites, but absence for M. mexicanus is correlated with its flanged syntergum and confirmation of absence for M. platyscapus requires additional, critical-point dried females. The presence of a distinct supra-anal plate in brachypterous females of most species here classified as the excavatus -group of Merostenus indicates that their superficially plesiomorphic syntergal structure actually is a derived structure that evolved though loss of a syntergal emargination in association with the ovipositor sheaths being secondarily shortened, but with retention of the supra-anal plate in most species, even though sometimes it is very small. A reduced syntergal emargination associated with comparatively short ovipositor sheaths but with retention of a distinct supra-anal plate along the posterior margin of the syntergum is exhibited by the fully winged females of at least one species of M. ( Merostenus ) ( Fig. 10 View FIGURES 9 – 14 ) and M. ( Reikosiella ) ( Fig. 9 View FIGURES 9 – 14 ). This indicates that reduction of a syntergal emargination is at least possible, if not likely, when the ovipositor sheaths are secondarily shortened in Reikosiella . Interestingly, no species of E. ( Macroneura ) or E. ( Eupelmus ) with brachypterous females have secondarily lost an omega-like emargination even though the ovipositor sheaths are comparatively short and rigid. This may be for some functional reason correlated with an extremely short rather than more extensive dorsal surface of the syntergum characteristic of Reikosiella and Merostenus sensu Gibson (1995) .
The syntergal structure of R. ( Incohata ) is at least very similar to that postulated as the groundplan eupelmine structure ( cf. Figs 1, 3 View FIGURES 1 – 8 ), though the subanal plate is comparatively large and suboval ( Fig. 4 View FIGURES 1 – 8 ), which is more similar to that of other Reikosiella and eupelmines rather than P. chilensis ( Fig. 2 View FIGURES 1 – 8 ). As a unique state, the very slender, only mediolongitudinally sclerotized subanal plate of P. chilensis could be an autapomorphy of that species or a uniquely retained symplesiomorphy within Eupelminae . If the latter, the more extensively sclerotized/melanized subanal plate represents another synapomorphy for Eupelminae excluding Phenaceupelmus . It also remains to be determined more confidently whether an apical line (e.g. Fig. 8 View FIGURES 1 – 8 ) rather than just 2 paramedial premarginal setae (e.g. Fig. 3 View FIGURES 1 – 8 ) is the groundplan syntergal setal pattern for R. ( Incohata ) and the genus. Critical-point dried females show that an anal tube can extend beyond the syntergum in R. ( Incohata ), and the membrane anterior to the anus is at least sometimes slightly sclerotized/melanized (yellowish) dorsally ( Fig. 3 View FIGURES 1 – 8 ). However, this region is not as strongly sclerotized/melanized as for females of the other three subgenera, in which it typically appears as quite a distinct sclerite along the posteromedial margin of the syntergum ( Figs 5–10 View FIGURES 1 – 8 View FIGURES 9 – 14 ). A basal position for R. ( Incohata ) relative to the other three subgenera is thus supported not only by structure of the sdp-complex of females ( Gibson 1995, fig. 189), but also by syntergal structure, i.e. lacking a deep syntergal emargination in combination with at most having a very slightly sclerotized/melanized supra-anal plate, and possibly a row of long premarginal setae as groundplan features. The presence of a comparatively much more strongly sclerotized/melanized supra-anal plate in females of the other three subgenera of Reikosiella ( Figs 5–10 View FIGURES 1 – 8 View FIGURES 9 – 14 ) and a variably strongly sclerotized/melanized supra-anal plate in other basal genera characterized by a deeply emarginate syntergum ( Figs 11–18 View FIGURES 9 – 14 View FIGURES 15 – 21. 15 – 18 ) suggests that the membrane dorsal to the anus is prone to secondary sclerotization/melanization for some functional reason when the anal tube is directed more posterodorsally to dorsally than posteriorly to posteroventrally. Most likely this is to lessen desiccation and/or for protection of the secondarily exposed anus. Based on syntergal structure of R. ( Incohata ), sclerotization/melanization of the supra-anal plate in the other three subgenera of Reikosiella ( Figs 5– 10 View FIGURES 1 – 8 View FIGURES 9 – 14 ) is indicated to have evolved independently of that of Australoodera ( Fig. 11 View FIGURES 9 – 14 ), Ecnomocephala ( Fig. 13 View FIGURES 9 – 14 ), Eupelmus ( Figs 15–18 View FIGURES 15 – 21. 15 – 18 ), Phlebopenes ( Fig. 14 View FIGURES 9 – 14 ) and Tineobius ( Fig. 12 View FIGURES 9 – 14 ). Further, it is only within R. ( Capreocauda ) ( Fig. 5 View FIGURES 1 – 8 ) + R. ( Hirticauda ) ( Figs 6 View FIGURES 1 – 8 , 10 View FIGURES 9 – 14 ) + R. ( Reikosiella ) ( Figs 7–9 View FIGURES 1 – 8 View FIGURES 9 – 14 ) sensu Gibson (1995) , most species of what is here classified as the excavatus -group of Merostenus ( Figs 47, 48 View FIGURES 39 – 48 , 105 View FIGURES 98 – 105 , 122 View FIGURES 115 – 123 ), and at least some species of T. ( Tineobius ) ( Fig. 12 View FIGURES 9 – 14 ) and the hartigi -group of E. ( Episolindelia ) ( Figs 17, 18 View FIGURES 15 – 21. 15 – 18 ; Gibson 1995, fig. 320) that the supraanal plate is so strongly sclerotized/melanized that it appears as a distinct sclerite posterior to the syntergal emargination.
Eupelmus View in CoL + Australoodera View in CoL + Tineobius View in CoL are indicated as a monophyletic group based on shared presence of a comparatively large and light-colored frontal prepectal surface ( Gibson 1995, character 19, state 2), though females of Mesocomys View in CoL , which have a syntergal flange, have a similar frontal prepectal surface structure ( Gibson, 1995, table 1). Ecnomocephala View in CoL + Eupelmus View in CoL + Australoodera View in CoL + Tineobius View in CoL may also be indicated as a monophyletic lineage by common reduction of the dorsal surface of the syntergum to a slender band anterior to the emargination ( Figs 11–13 View FIGURES 9 – 14 ). Reduction of the syntergal premarginal setae to at most two long setae paramedially may also be a synapomorphy for the four genera, though it remains to be proven whether a similar setal pattern in some Reikosiella View in CoL results from common ancestry or homoplasy. The reduction in number of syntergal premarginal setae to just two setae paramedially perhaps is prone to evolve for some functional reason in taxa with a deeply emarginate syntergum and sclerotized supra-anal plate, possibly used to sense the angle of the supra-anal plate when the anal tube is extended during defecation. However, as noted above, females of at least a couple of species of Calymmochilus View in CoL have a similar setal pattern even though they lack a supra-anal plate and not all females with a deeply emarginate syntergum and noticeably sclerotized supra-anal plate have two syntergal premarginal setae paramedially. The syntergal emargination of Phlebopenes View in CoL is almost certainly independently derived from the other five basal genera with an emargination because not only is the dorsal surface of the syntergum extensive anterior to the emargination but there is a line of long premarginal setae that project over the emargination ( Fig. 14 View FIGURES 9 – 14 ) similar to those few Brasema View in CoL with a deeply emarginate syntergum ( Fig. 20 View FIGURES 15 – 21. 15 – 18 ). The functional advantage of a deeply emarginate syntergum remains uncertain. Females with the plesiomorphic syntergal structure ( Figs 1, 3 View FIGURES 1 – 8 , 19 View FIGURES 15 – 21. 15 – 18 ) or those with a syntergal flange apparently have the anus directed posteriorly to posteroventrally over the ovipositor sheaths for defecation, whereas those with a deeply emarginate syntergum have the anus directed posterodorsally to dorsally. This is taken to the most extreme condition in some E. ( Episolindelia) hartigi -group species ( Fig. 18 View FIGURES 15 – 21. 15 – 18 ). One would think it would be a disadvantage to defecate dorsally over one’s self ( Fig. 18 View FIGURES 15 – 21. 15 – 18 ), but it might be advantageous to lessen chances of fouling the ovipositor.
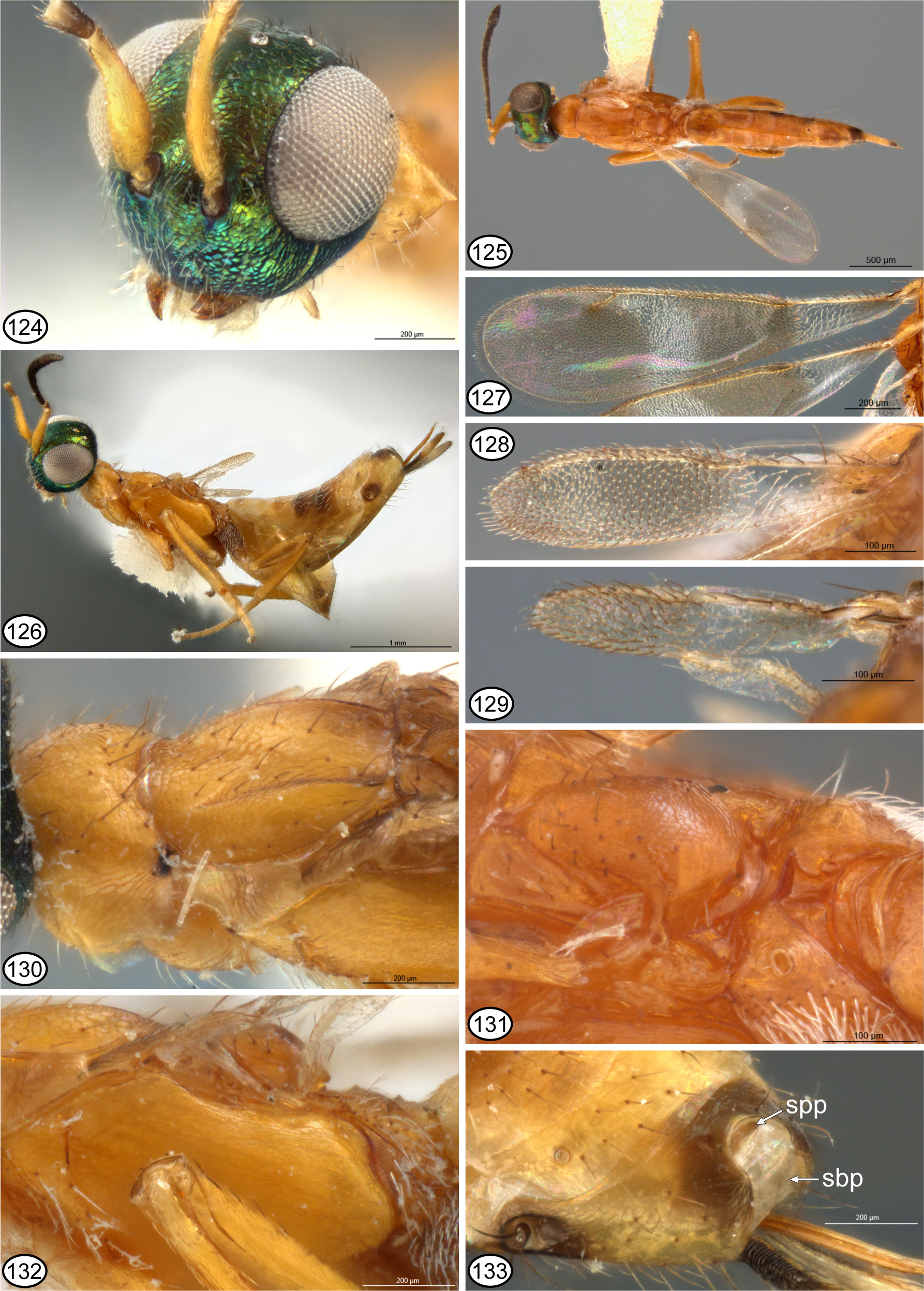
Other than what was interpreted as the plesiomorphic syntergal structure, Gibson (1995) retained Merostenus View in CoL as a valid genus primarily because females exhibit the following three features: brachyptery (character 29, state 2), pronotum completely sclerotized (character 14, state 2), and scutellum not extending to transscutal articulation such that the axillae are contiguous anteriorly but are separated by a variably distinct depression anterior to the scutellum (structure not analyzed as a character). However, all of these features are possessed also by females of some species of Reikosiella sensu Gibson (1995) View in CoL . Wing polymorphism in M. ( Reikosiella) melinus and female brachyptery in the two non- excavatus View in CoL group species of M. ( Merostenus View in CoL ), M. congoensis and M. longistylus , indicate brachyptery evolved at least twice and, depending on the phylogenetic relationships of the latter two species with those of the excavatus View in CoL species-group, possibly more times in Merostenus View in CoL in the present sense. Furthermore, a somewhat differently sculptured and/or lighter-colored mediolongitudinal line anteriorly on the pronotum of most M. excavatus View in CoL females ( Fig. 41 View FIGURES 39 – 48 ) and a slight mediolongitudinal depression posteriorly on the pronotum of the unique holotype of M. reticulatus ( Fig. 100 View FIGURES 98 – 105 ) indicates a completely sclerotized pronotum in the excavatus View in CoL species-group evolved through secondary sclerotization from one or more ancestors that had the pronotum divided medially. Except for one species of Xenanastatus View in CoL , females of no other eupelmine genera are known to have a completely sclerotized pronotum other than some fully winged species of R. ( Capreocauda ) and R. ( Reikosiella) sensu Gibson (1995, table 1, character 14) , including both macropterous and brachypterous females of M. melinus ( Figs 125, 130 View FIGURES 124 – 133 ). This demonstrates that secondary pronotal sclerotization, like female brachyptery, evolved at least twice in Merostenus View in CoL in the present sense. I have not seen any macropterous females of R. ( Hirticauda) sensu Gibson (1995) with a completely sclerotized pronotum. If Merostenus View in CoL represents only a modified subgroup of the latter subgenus then a completely sclerotized pronotum evolved independently in all recognized subgenera except M. ( Incohata ). Finally, females of M. ( Reikosiella) melinus and of the two nonexcavatus group species of M. ( Merostenus View in CoL ) all have scutellar-axillar structures in which the axillae are separated by a variably large and distinct depression anterior to the base of the scutellum ( Figs 26 View FIGURES 22 – 30 , 61, 63 View FIGURES 56 – 65 ), though certainly this is very small and inconspicuous for M. melinus ( Figs 130, 131 View FIGURES 124 – 133 ). In addition, females of all species classified in the excavatus View in CoL species-group have a modified mesoscutal structure in which the anterior margin is variably upcurved anterior to the lateral lobes ( Figs 33 View FIGURES 31 – 38 , 41 View FIGURES 39 – 48 , 86 View FIGURES 83 – 89 , 93 View FIGURES 90 – 97 , 100 View FIGURES 98 – 105 ). Females of M. melinus ( Fig. 130 View FIGURES 124 – 133 ) and M. longistylus ( Fig. 60 View FIGURES 56 – 65 ) do not have such a modified mesoscutum, but females of M. congoensis do ( Fig. 26 View FIGURES 22 – 30 ).
Consequently, none of the features possessed by females of species that would be classified in Merostenus sensu Gibson (1995) are unique to the group, but are shared also with some species that would be classified in one or more subgenera of Reikosiella sensu Gibson (1995) . Further, as given in the diagnosis for M. ( Merostenus ) below, females that would be classified in Merostenus and R. ( Hirticauda ) share presence of mesotibial apical pegs and other putatively derived structures of the sdp-complex and acropleuron.
Of the seven species here classified as the excavatus -group of Merostenus , only males of M. excavatus are definitely associated with females through multiple rearing events. However, the males of M. reticulatus and of the non- excavatus group species M. longistylus are newly described based on association with females through collecting. Fusu (2008) determined that M. excavatus has a karyotype similar to what Fusu (2013) classified as R. ( Hirticauda) rostrata and different from that of species of Eupelmus . This at least supports a possible phylogenetic association of M. excavatus and Reikosiella , though not that Merostenus renders Reikosiella paraphyletic. Gibson (1995) further stated that males of M. excavatus have a small pit or circular depression within the scrobal depression at the apex of the interantennal prominence (character 47, state 3) similar to many males of Reikosiella sensu Gibson (1995, figs 406–408) . Fusu (2013, figs 43, 44) illustrated a distinct facial pit for one M. excavatus male, though the pit is at best obscure in many individuals ( Fig. 51 View FIGURES 49 – 55 ). Even though apparently variable in development, presence of an evident pit for at least some M. excavatus males further supports at least a sister-group relationship between Merostenus and Reikosiella sensu Gibson (1995) . The lack of an evident pit from males of both M. longistylus ( Fig. 68 View FIGURES 66 – 73 ) and M. reticulatus ( Figs 108, 109 View FIGURES 106 – 114 ) demonstrates presence or absence of a distinct scrobal depression pit is variable for both Reikosiella and Merostenus sensu Gibson (1995) .
Fusu (2013, fig. 44) differentiated males of M. excavatus from regional males of R. ( Hirticauda ) by Gt1 being contrastingly dark yellow to pale brownish relative to the remainder of the gaster and the fore wings being slightly, uniformly infuscate. The male of M. reticulatus also has slightly infuscate fore wings ( Fig. 113 View FIGURES 106 – 114 ) and the gaster partly pale at least ventrally ( Fig. 107 View FIGURES 106 – 114 ). However, smaller males of M. excavatus do not have the fore wings noticeably infuscate ( Fig. 53 View FIGURES 49 – 55 ). More comprehensive character-state analysis is required to assess accurately, but fore wing and gastral color patterns likely are more of species than generic features. Gibson (1995, couplet 54 in key to males) questionably differentiated M. excavatus males from those of Reikosiella based on the metapleuron extending only about two-thirds or less the distance to the base of the propodeum, and the mesepimeron having the posterodorsal margin raised as slender flange lateral to the base of the propodeum ( Gibson 1995, cf. figs 434, 436). Perhaps a more objective comparison is that in males of M. excavatus the metapleuron extends dorsally only to about the level of the anterior margin of the spiracle ( Figs 50 View FIGURES 49 – 55 : insert, 55). The males of M. reticulatus and M. longistylus have the metapleuron extending more dorsally, obviously anterior to the level of the propodeal spiracle ( Figs 71 View FIGURES 66 – 73 , 111 View FIGURES 106 – 114 ), though both have the posterodorsal margin of the acropleuron developed as a slender flange that is separated from and raised slightly over the anterodorsal margin of the metapleuron. Character-state analysis of this feature across Eupelminae is necessary to assess its phylogenetic significance, but at least some male Eupelmus also have the posterodorsal margin of the acropleuron separated from the anterodorsal margin of the metapleuron. Consequently, this structure is likely symplesiomorphic at the level of Reikosiella sensu Gibson (1995) and the metapleural structure of M. excavatus probably represents a secondarily more derived, species feature.
The males of very few species of Reikosiella sensu Gibson (1995) are known through association with females so that limits of variation remain uncertain. Gibson (1995) diagnosed and keyed four groups of males in Reikosiella based on flagellar structure and setation. Subsequent study indicates that “flagellar type 3” ( Gibson 1995, fig. 370) and “flagellar type 4” ( Gibson 1995, fig. 371) do not belong to males of Reikosiella sensu Gibson (1995) , but to males of Ooderella Ashmead (these results to be published separately). However, “flagellar type 1” ( Gibson 1995, figs 367, 368; Fusu 2013, figs 22, 27, 30) and “flagellar type 2” ( Gibson 1995, fig. 369) are possessed by males of Reikosiella sensu Gibson (1995) . The flagellum of the latter two types are characterized as being variably long but comparatively gracile-filiform with funiculars that are obviously longer than wide and with comparatively long setae at least about as long as the width of a funicular and with variably long mps that, if appearing dense, are quite long and at most in widely overlapping rather than short and in multiple rows per funicular. Type 3 and type 4 flagella differ by having very short and inconspicuous setae; further, the mps are dense and either comparatively short and in multiple rows per funicular (type 3) or each has a long, slender, apically free portion such that the mps superficially look like setae projecting apically at an acute angle relative to the funicle (type 4). Fusu (2013, figs 22, 27, 30) also noted that at least Palaearctic Reikosiella males have the funiculars separated by short pedicels as compared to Eupelmus males in which the funiculars abut (e.g. Gibson and Fusu 2016, figs 9f, 12g, 14d, 23h).
Although not all New World males considered to belong to Merostenus in the present sense have the funiculars separated by evident pedicels, those of M. longistylus ( Fig. 69 View FIGURES 66 – 73 ), M. excavatus ( Fig. 52 View FIGURES 49 – 55 ) and M. reticulatus ( Fig. 110 View FIGURES 106 – 114 ) do, which also supports at least a sister-group relationship between Merostenus and Reikosiella sensu Gibson (1995) . Males of the latter two excavatus -group species have the funiculars conspicuously longer than most males of Reikosiella sensu Gibson (1995, figs 368, 369) or Fusu (2013, figs 22, 30) or M. longistylus ( Fig. 69 View FIGURES 66 – 73 ). However, some Afrotropical males associated through collection records with females that would be classified in R. ( Hirticauda) sensu Gibson (1995) have similarly long and setose funiculars as those of M. excavatus and M. reticulatus . If the sex associations are correct they indicate funicular length is variable for males of Reikosiella sensu Gibson (1995) . Gibson (1995) hypothesized that the plesiomorphic male flagellar structure for Eupelminae (character 50, state 1) was a subclavate flagellum composed of comparatively short funiculars with outstanding setae and relatively sparse mps similar to that of P. chilensis males ( Gibson 1995, fig. 349). Males of Australoodera , Ecnomocephala and Tineobius have the flagellum variably more distinctly clavate. Although flagellar structure is highly variable in Eupelmus ( Gibson 2011; Gibson and Fusu 2016), a clavate flagellum is the most likely groundplan structure based on typical species of E. ( Episolindelia ) ( Gibson 1995, fig. 351). Males of Phlebopenes have a compact-filiform to lobate flagellum ( Gibson 1995, fig. 343). Consequently, a comparatively long and gracile-filiform flagellum with outstanding setae and comparatively sparse though variably long mps further supports a hypothesis that Merostenus + Reikosiella sensu Gibson (1995) constitute a monophyletic group, though not necessarily that Merostenus renders Reikosiella paraphyletic. Further character-state analysis is required to determine whether or not funicular pedicels are a groundplan feature of the male antenna of Reikosiella sensu Gibson (1995) , particularly those of R. ( Incohata ). If not, presence of funicular pedicels would more strongly support the hypothesis that Merostenus renders Reikosiella paraphyletic.
Gibson (1995) stated that the fore wings of male Reikosiella are “often” distinctively long with the marginal vein “usually” at least 0.7× the width of the wing and the disc “usually” entirely setose though sometimes with an indistinct bare region below the parastigma and base of the marginal vein. This latter statement is inaccurate because many Reikosiella males, particularly ones with a distinctively elongate-narrow fore wing and long marginal vein, have the disc variably extensively bare dorsally behind the base of the marginal vein and parastigma to the basal and mediocubital folds. This bare region is less distinct than the more conspicuous speculum that characterizes male Eupelmus (see figures in Gibson 2011 and Gibson and Fusu 2016) because unlike in Eupelmus it is partly obscured by setae on the ventral surface. However, the dorsally bare setal pattern could represent an intermediate state in a transformation series resulting in the development of both a dorsally and ventrally bare region. If so, a possible sister-group relationship between Merostenus in the present sense and Eupelmus is indicated. However, most males now identified as Ooderella also have a dorsally bare region behind the parastigma and base of the marginal vein that is more or less obscured by setae on the ventral surface (results to be published separately). Further, males are not associated with females for any M. ( Incohata ) species so that the groundplan fore wing structure, setal pattern, and venation all remain unsubstantiated for Merostenus .
Fusu (2013) recognized the males of five species of R. ( Hirticauda ) in the Palaearctic, R. hungarica , R. koreana Fusu , R. rostrata , R. tripotinorum Fusu and R. vanharteni Fusu. All have entirely setose fore wings or with at most a small, indistinct area of sparse setae behind the parastigma ( Fusu 2013). Fore wing length varies between about 2.2–2.5× wing width, and length of the marginal vein between about 0.63–0.76× wing width. Fore wings of the newly described non- excavatus group species M. longistylus are similar in being less than 2.5× as long as wide, and with the marginal vein only about 0.6× the width of the wing, but they uniquely have an oblique bare band behind the parastigma and base of the marginal vein that is separated by setae from the venation and basal and mediocubital folds ( Fig. 72 View FIGURES 66 – 73 ). This bare band thus resembles a linea calva, which characterizes most fully winged E. ( Eupelmus ) females. The fore wings of male M. excavatus ( Fig. 53 View FIGURES 49 – 55 ) and M. reticulatus ( Fig. 113 View FIGURES 106 – 114 ), the only two excavatus -group species for which males are known, are entirely setose though conspicuously elongate-slender with a comparatively long marginal vein (fore wing length at least about 2.9× width and marginal vein at least about 0.9× wing width). Based merely on these observations, male fore wing structure and venation might be considered as putative synapomorphies for the excavatus -group. However, many, if not most New World Reikosiella males sensu Gibson (1995) have elongate-slender fore wings with comparatively long marginal veins more similar to those of M. excavatus and M. reticulatus . Although unassociated with females, such males are almost certainly those of R. ( Reikosiella) sensu Gibson (1995) . Further, at least some males from the Afrotropical and Oriental regions, which probably are males of R. ( Hirticauda ) or R. ( Capreocauda) sensu Gibson (1995) , have similarly long wings and marginal veins. Consequently, if comparatively short and broad fore wings with a comparatively short marginal vein represent groundplan features of Merostenus in the present sense, and if the different subgenera are monophyletic clades, then elongation of the fore wing and marginal vein appear to have evolved convergently in at least M. ( Reikosiella ) and M. ( Merostenus ). Finally, Fusu (2013) described the gaster of males of three of five species of R. ( Hirticauda ) from the Palaearctic as being compressed basally, similar to the condition of M. reticulatus ( Fig. 106 View FIGURES 106 – 114 ), but not M. excavatus or M. longistylus , which have a transversely oval to somewhat flattened gaster ( Figs 49 View FIGURES 49 – 55 , 66 View FIGURES 66 – 73 ). The presence of the same state in multiple males of the same species demonstrates the difference is not simply an artefact of preservation but is taxonomically significant. However, presence of the two different male gastral structures for species with fully winged females as well as within the excavatus -group indicates multiple origins or losses of a basally compressed gaster. Males have yet to be associated with females for any species here classified in M. ( Incohata ) or M. ( Capreocauda ). It remains to be shown through association of the sexes of many more species than at present whether the four subgenera recognized in Merostenus can be differentiated by male as well as female features. At present, presence or absence of an evident pit within the scrobal depression, flagellar structure, fore wing features, and gastral structure all appear to be homoplastic.
Based on the above character analysis for females and males, several congruent states at least support Merostenus + Reikosiella sensu Gibson (1995) as a monophyletic group. The presence of a comparatively strongly sclerotized/melanized supra-anal plate for females of most species that would be classified in Merostenus ( Figs 38 View FIGURES 31 – 38 , 48 View FIGURES 39 – 48 , 89 View FIGURES 83 – 89 , 105 View FIGURES 98 – 105 , 122 View FIGURES 115 – 123 ) and Reikosiella ( Figs 5–10 View FIGURES 1 – 8 View FIGURES 9 – 14 , 30 View FIGURES 22 – 30 , 65 View FIGURES 56 – 65 , 133 View FIGURES 124 – 133 ) sensu Gibson (1995) excluding the hypothesized most basal group, R. ( Incohata ) ( Fig. 3 View FIGURES 1 – 8 ), additionally supports a hypothesis that Merostenus renders Reikosiella paraphyletic. Presence of mesotibial apical pegs and similar structures of the sdp-complex and acropleuron also indicate Merostenus renders R. ( Hirticauda ) paraphyletic. I conclude that Merostenus is nothing more than a small group of species of Reikosiella sensu Gibson (1995) whose females share a suite of conspicuously modified features, which may have evolved convergently more than once (see further below). These include the loss of a deep syntergal emargination as a consequence of the ovipositor sheaths being secondarily shortened, but in most species with retention of an evident supra-anal plate along the posterior margin of the syntergum as evidence of an ancestral deeply emarginate syntergum. It also includes secondary reduction of the wings (brachyptery) and medial sclerotization of the pronotum. I therefore newly synonymize Reikosiella Yoshimoto, 1969 under Merostenus Walker, 1837 , treat Reikosiella ( Hirticauda Bouček 1988 ) as a junior synonym of M. ( Merostenus ), and recognize M. ( Capreocauda Gibson 1995 ), M. ( Incohata Gibson 1995 ) and M. ( Reikosiella ) as subgenera within Merostenus . I also treat as the excavatus species-group within M. ( Merostenus ) those species whose females are brachypterous and have a uniformly sclerotized pronotum.
Relationships of Merostenus with the other genera considered as basal within Eupelminae by Gibson (1995), particularly those with a deeply emarginate syntergum, remain to be clarified. Such putatively apomorphic features as a reduced dorsal surface of the syntergum anterior to the emargination and a comparatively large, pale anterior prepectal surface support a possible Ecnomocephala + ( Eupelmus + Australoodera + Tineobius ) sister-group relationship. However, both females and males of some species of Merostenus share similarities with some Eupelmus , particularly species of E. ( Episolindelia ), which is hypothesized to represent a grade of the more basal species of Eupelmus ( Gibson 1995; Gibson & Fusu 2016). Further, some species of Merostenus exhibit putatively plesiomorphic and others apomorphic structures of the sdp-complex, mesotrochantinal plate, and syntergal structure. The similarities between some Merostenus and Eupelmus might all represent symplesiomorphies and, if so, could support Merostenus as the sister-group of at least Eupelmus + Australoodera + Tineobius . Other than perhaps a comparatively elongate-slender fore wing and long marginal vein in females, substantial support for monophyly of Merostenus is lacking. Additional phylogenetic analysis supported by molecular evidence is required to document that the four subgenera recognized in Merostenus constitute a monophyletic lineage and to confidently resolve relationships with other eupelmine genera.
Monophyly and relationships of the excavatus species-group. The excavatus species-group within M. ( Merostenus ) is here established for seven species ( M. distigma , M. excavatus , M. mexicanus , M. micropterus , M. platyscapus , M. reticulatus and M. speculum ) that under the concepts of Gibson (1995) would have been classified in Merostenus based on a combination of four features (female brachyptery, completely sclerotized pronotum, medially separated axillae anterior to the scutellum, and superficially unmodified syntergum in all but M. mexicanus ). Monophyly of the excavatus -group is not supported by any uniquely shared features, and because of their uniquely flanged syntergum possible relationships of M. mexicanus to the other excavatus -group species are particularly intriguing. Females of two non- excavatus group species within M. ( Merostenus ), M. congoensis and M. longistylus , are brachypterous, which could support one or both as sister taxa of the excavatus group. However, brachyptery evolved at least twice in Merostenus because it also evolved in M. ( Reikosiella) melinus . Brachyptery may therefore have also evolved more than once in M. ( Merostenus ). As noted above, possible monophyly of M. congoensis + excavatus -group species is supported by one shared feature—the anterior margin of the mesoscutum being variably distinctly upcurved behind the pronotum in front of the lateral lobes (excluding possibly M. mexicanus , see description). Females of M. longistylus and M. melinus , the other two non- excavatus group species with brachypterous females do not have an anteriorly modified mesoscutum. Females of M. congoensis also have the syntergum only slightly incurved ( Fig. 29 View FIGURES 22 – 30 ) relative to the more distinctly incurved synterga of M. melinus ( Fig. 133 View FIGURES 124 – 133 ) and M. longistylus ( Fig. 65 View FIGURES 56 – 65 ), which might represent an intermediate state in evolution of the non-emarginate synterga of excavatus -group females. Therefore, M. congoensis is indicated as the most likely sister group of the excavatus group based on shared female brachyptery, an anteriorly upcurved mesoscutum, and a reduced syntergal emargination in association with strongly reduced ovipositor sheaths.
Within the excavatus -group, four of five Afrotropical species ( M. distigma , M. micropterus , M. reticulatus and M. speculum ) are supported as a monophyletic clade. Females of these four species share a coriaceous to coriaceous-imbricate scutellar-axillar complex that consists of a uniformly convex and oval scutellum and elongate-slender axillae separated by a deep depression anterior to the scutellum ( Figs 36 View FIGURES 31 – 38 , 87 View FIGURES 83 – 89 , 103 View FIGURES 98 – 105 , 119 View FIGURES 115 – 123 ), an elongate propodeum with the foramen shallowly incurved and with the panels variably distinctly concave on either side of a low median carina ( Figs 36 View FIGURES 31 – 38 , 87 View FIGURES 83 – 89 , 103 View FIGURES 98 – 105 , 119 View FIGURES 115 – 123 ), and a mostly setose and similarly structured metapleuron ( Figs 35 View FIGURES 31 – 38 , 88 View FIGURES 83 – 89 , 102 View FIGURES 98 – 105 , 120 View FIGURES 115 – 123 ). All of these shared features likely are synapomorphic for the four Afrotropical species, though their shared scutellar-axillar structure shares some features with non- excavatus group species. An evenly convex and uniformly oval scutellum is shared with M. melinus ( Fig. 131 View FIGURES 124 – 133 ) and M. congoensis ( Fig. 28 View FIGURES 22 – 30 ), whereas elongateslender axillae that are separated by a comparatively large and deep depression is shared with M. longistylus ( Fig. 61 View FIGURES 56 – 65 ). A convex, more or less oval scutellum is likely plesiomorphic but a large depression separating the axillae is undoubtedly apomorphic. In addition to their axillar structure, the scutellar structure of M. longistylus is likely also derived, being comparatively broad and convex posteriorly but tapered anteriorly with inclined sides so as to form a dorsolongitudinal angulation ( Figs 61, 63 View FIGURES 56 – 65 ). This structure is similar to that of the excavatus -group species M. excavatus ( Fig. 43 View FIGURES 39 – 48 ), M. mexicanus ( Fig. 78 View FIGURES 74 – 82 ) and M. platyscapus ( Fig. 94 View FIGURES 90 – 97 ) except that in the latter three the scutellum is at least partly carinately margined dorsolongitudinally. Females of M. platyscapus also have the axillae longitudinally carinate on either side of an anteriorly carinate scutellum ( Fig. 94 View FIGURES 90 – 97 ), whereas females of M. excavatus have large, entirely longitudinally striate-strigose axillae anterior to a much less distinctly anteriorly carinate scutellum ( Fig. 43 View FIGURES 39 – 48 ).
Females of the four Afrotropical species and M. melinus not only share a likely plesiomorphic scutellar structure, but also a setose though differently structured metapleuron. In the four Afrotropical species the metapleuron is virtually entirely setose except the dorsal half to two-thirds of the anterior margin is at least linearly bare and slightly curved anteriorly or reflexed into a slender flange along the posterodorsal margin of the acropleuron. The setose anteroventral margin is also variably distinctly angled posteroventrally above the base of the mesocoxa, and the ventral margin above the metacoxa is only slightly reflexed such that a distinct ventral region is not differentiated between the meso- and metacoxae ( Figs 35 View FIGURES 31 – 38 , 88 View FIGURES 83 – 89 , 102 View FIGURES 98 – 105 , 120 View FIGURES 115 – 123 ). Females of M. melinus differ by having the metapleuron bare dorsally, flat, and with the anterior margin slightly sinuate so that medially it forms an anteriorly curved region that fits within the emarginate posterodorsal margin of the acropleuron ( Fig. 132 View FIGURES 124 – 133 ); a slender, bare, ventral region is present between the acropleuron and base of the metacoxa. Gibson (1995) hypothesized that a setose metapleuron is plesiomorphic within Eupelminae . Although a revision of Merostenus is required to determine exact character-state distribution, females of the other species treated here and the vast majority of other species in the genus have a bare metapleuron, including those of M. ( Incohata ). Consequently, the setose metapleuron in M. melinus and the four Afrotropical species likely represent independent origins in M. melinus and in the common ancestor of the Afrotropical species, though females of M. excavatus and M. platyscapus might also have a partly setose, albeit highly modified metapleuron. Most examined museum females of M. excavatus have one ( Fig. 44 View FIGURES 39 – 48 : arrow) or two setae on a ventrally faced region differentiated between the acropleuron, recurved ventral margin of the metapleuron, and base of the metacoxa. Males also typically have one or two setae in a similar position, but within the anteroventral angle of the metapleuron itself ( Fig. 50 View FIGURES 49 – 55 : insert). Females of M. platyscapus have a similarly differentiated ventral region as females of M. excavatus but it is much more extensively setose ( Fig. 95 View FIGURES 90 – 97 ). Males of M. platyscapus are unknown, but they too may have an anteroventrally setose metapleuron based on both sexes of M. excavatus and M. reticulatus having similar setal patterns. If so, this might indicate that the ventral region bearing the setae in female M. excavatus and M. platyscapus actually represents a secondarily demarcated part of the metapleuron. Females of the other described brachypterous species have quite differently structured metapleura. In M. longistylus ( Fig. 62 View FIGURES 56 – 65 ) the anterior margin is abruptly sinuate at about mid-height so that the metapleuron is sublinear dorsally but much wider over about the ventral half where it is slightly reflexed as a comparatively inconspicuous slender flange. In an uncontorted state this slender flange extends over a depressed region of the acropleuron along its posterodorsal margin, whereas the ventral margin is more distinctly reflexed into a slender flange over the base of the mesocoxa so as to differentiate a small, bare, ventral region between the acropleuron and basal margin of the metacoxa. Females of M. congoensis ( Fig. 25 View FIGURES 22 – 30 ), M. excavatus ( Fig. 44 View FIGURES 39 – 48 ) and M. platyscapus ( Fig. 95 View FIGURES 90 – 97 ) also have the anterior margin of the metapleuron variably extensively reflexed into a slender flange abutting the posterior margin of the acropleuron, with the entire anterior margin reflexed in M. congoensis and M. platyscapus , but only about the ventral third reflexed for M. excavatus . In all three species the ventral margin is reflexed above the metacoxa so that a ventral region is differentiated between the acropleuron and metacoxal base. This latter region is not clearly visible in the holotype of M. congoensis because of glue, but it appears to be bare or at most as sparsely setose as for M. excavatus . Because of its contorted state, exact structure of the metapleuron is also not clearly visible for the holotype of M. mexicanus , but it is at least bare. As a result of contortion ( Fig. 74 View FIGURES 74 – 82 ) the propodeum and metapleuron are angled upwards relative to the posterior margin of the acropleuron ( Fig. 80 View FIGURES 74 – 82 ) such that the anterior margin of the metapleuron is horizontal rather than vertical behind the acropleuron. The left side is covered in glue, but the right side has dark spots below the horizontal anterior margin ( Fig. 80 View FIGURES 74 – 82 ), which might indicate a setose ventral region, though this needs to be confirmed by additional specimens. In a female of M. excavatus in which the metapleuron is slightly separated from the acropleuron, the anterior part is seen to form a concave ‘pocket’ into which the posterior margin of the acropleuron normally fits. Gibson (1989) hypothesized that some sort of mesothoracic locking mechanism was necessary for the modified jumping structure of female eupelmines so that the acropleural (pl2-t2c) muscles can fully contract and stretch the resilin pad within each acropleuron prior to the stored energy being suddenly released to arch the mesonotum and pull up on the t2-tr2 muscles for jumping. Gibson (1995) subsequently hypothesized that this locking mechanism was formed by the conjunction between the anterior margin of the metapleuron and the variably incised posterior margin of the acropleuron. Because of the variation exhibited in metapleural structure within just the few species of Merostenus treated here, a revision of the genus is required to determine the most likely groundplan structure for the genus. However, based on M. ( Incohata ) females, the groundplan structure most likely was similar to that of M. longistylus ( Fig. 62 View FIGURES 56 – 65 ). Regardless, except for the four Afrotropical excavatus -group species discussed above, the other treated species do not share multiple, congruent, putatively derived features that would strongly support sister-group relationships. Molecular methods should help resolve such relationships.
| CNC |
Canadian National Collection of Insects, Arachnids, and Nematodes |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Chalcidoidea |
|
Family |
Merostenus Walker
| Gibson, Gary A. P. 2017 |
Reikosiella ( Capreocauda )
| Gibson 1995: 262 |
Reikosiella ( Incohata )
| Gibson 1995: 263 |
Reikosiella ( Reikosiella )
| Gibson 1995: 265 |
Reikosiella ( Hirticauda )
| Gibson 1995: 263 |
Hirticauda Bouček, 1988 : 558
| Gibson 1995: 259 |
| Boucek 1988: 558 |
Reikosiella
| Yoshimoto 1969: 627 |
Finlayia
| Boucek 1988: 558 |
| Girault 1934: 1 |
| Giles 1904: 365 |
Eupelminus
| Ruschka 1921: 309 |
| Dalla 1897: 85 |
Urocryptus
| Ruschka 1921: 309 |
| Dalla 1897: 85 |
| Westwood 1839: 72 |
| Temminck 1838: 31 |
| Dalman 1820: 382 |
Merostenus
| Walker 1837: 354 |