Trichomycterus striatus ( Meek & Hildebrand, 1913 )
|
publication ID |
https://doi.org/10.11646/zootaxa.4420.4.5 |
|
publication LSID |
lsid:zoobank.org:pub:03CF68A6-7A81-487E-A33F-1777036302B3 |
|
DOI |
https://doi.org/10.5281/zenodo.5986544 |
|
persistent identifier |
https://treatment.plazi.org/id/03C287D1-3808-FFC3-5AA3-7A7BCA2D49AB |
|
treatment provided by |
Plazi |
|
scientific name |
Trichomycterus striatus ( Meek & Hildebrand, 1913 ) |
| status |
|
Trichomycterus striatus ( Meek & Hildebrand, 1913) View in CoL
Table 1; Figs. 1–7 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 .
Pygidium striatum Meek & Hildebrand, 1913: 78 View in CoL [ type locality: “Rio Cana, Cana”, Tuyra River basin, Pacific slope of Panama] ( Fig. 1A View FIGURE 1 ).— Meek & Hildebrand, 1916: 266 [brief description with notes on ecology and geographic distribution].— Eigenmann, 1918: 221 [in part; identification key; brief description with notes on ecology and distribution].— Eigenmann, 1922: 60 [in part; identification key; listed for Panama and Colombia].
Trichomycterus striatus Bussing, 1987: 110 View in CoL [brief description with notes on ecology and distribution].— Ibarra & Stewart, 1987: 12 [type catalog].— Burgess, 1989: 323 [in part; listed for southern Central America and Colombia; geographic and vertical distribution].— Ferraris & Vari 1992: 42 [type catalog].— Kramer & Bryant, 1995: 129 [diet, Panama].— Bussing, 1998: 159 [brief description with notes on ecology and geographic and vertical distribution].—de Pinna & Wosiacki, 2003: 285 [in part; listed for southern Central America and Colombia; geographic and vertical distribution].— Smith & Bermingham, 2005: 1840 [listed for Panama; geographic distribution].— Ferraris, 2007: 424 [in part; listed for southern Central America and Colombia; geographic and vertical distribution].—Angulo et al., 2013: 993 [listed for Costa Rica; geographic and vertical distribution].
Pygidium septentrionale Behre, 1928: 309 View in CoL , pl. 18 [ type locality: “Quebrada Salao”, tributary of the Chiriquí del Tire River, Chiriquí River basin, Pacific slope of Panama, altitude about 4000 feet] ( Fig. 1B View FIGURE 1 ).— Henn, 1928: 81 [ type catalog].— Ibarra & Stewart, 1987: 73 [ type catalog].
Trichomycterus septentrionale Burgess, 1989: 323 View in CoL [listed for southern Central America].
Diagnosis. Trichomycterus striatus can be distinguished from most congeners by body color: yellowish to light brown with a black lateral band and/or small dark brown spots on sides or uniformly light brown ( vs. not as described above; see Discussion). In addition, T. striatus differs from all northeastern South American congeners, and possibly all other trichomycterines, by the following combination of characters: eyes relatively well developed, eye diameter 11.0–20.9% of HL [ vs. reduced (eye diameter usually less than 10.0% of HL) in T. gorgona , T. santanderensis , T. steindachneri , T. sketi , T. tetuanensis , and T. uisae ; or absent in T. sandovali ]; teeth conical ( vs. incisiform in T. chapmani , T. gorgona , T. latidens , T. stellatus , and T. transandianus ); teeth arranged in 3–4 rows in both jaws ( vs. two in T. banneaui , T. gorgona , T. maldonadoi , and T. transandianus ); anterior section of the infraorbital canal (sensory pores i1 and i3) present ( vs. absent in T. maldonadoi , T. romeroi , and T. transandianus ); presence of a pair of sensory pores s6 ( vs. a single medial pore in T. nigromaculatus ); opercular odontodes 11–23 ( vs. less than 11 in T. montesi , T. sandovali , T. santanderensis , and T. uisae ); interopercular odontodes 27–44 ( vs. less than 27 in T. caliensis , T. chapmani , T. gorgona , and T. maldonadoi ); cleithrum lamina pierced by several broad foramina ( vs. cleithrum not pierced in most species; except T. ballesterosi , T. cachiraensis , T. sandovali , T. steindachneri , and T. transandianus ; see DoNascimiento et al. 2014); pectoral-fin branched rays 7–8 (vs. 5–6 in T. stellatus ; 6 in T. caliensis , T. chapmani , T. retropinnis , T. latidens , T. steindachneri , and T. romeroi ; 9 in T. sketi , and T. sandovali ); free vertebrae 36–37 ( vs. less than 36 in T. banneaui , T. garciamarquezi , T. latistriatus , T. maldonadoi , T. manaurensis , T. sketi ; or more than 37 in T. ballesterosi , T. bogotensis , T. caliensis , T. chapmani , T. gorgona , T. kankuamo , T. nigromaculatus , T. steindachneri , and T. uisae ); ribs 12–14 ( vs. less than 12 in T. gorgona , T. stellatus , and T. tetuanensis ); and caudal fin rounded to truncate ( vs. emarginate in T. banneaui , and T. tetuanensis ).
Description. Morphometric data provided in Table 1. Body elongate, semi-cylindrical, becoming compressed towards caudal fin ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ). Head wide and depressed, lateral contour swollen by well-developed jaw muscles, square to trapezoid in dorsal view; dorsal profile of head straight, ventral profile straight to convex ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ). Dorsal profile of trunk convex from nape to dorsal-fin origin, marked by epaxialis; ventral profile straight ( Figs. 1– 3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ). Dorsal and ventral profile of caudal peduncle straight to slightly convex ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ). Greatest body depth at mid-length of trunk; depth uniform posteriorly toward caudal fin. Skin of body with minute papillae, visible only under stereomicroscope.
Snout blunt. Mouth subterminal. Maxilla boomerang shaped ( Fig. 4 View FIGURE 4 ). Premaxilla rectangular, with three or four irregular rows of conical teeth ( Fig. 4 View FIGURE 4 ). Dentary with three or four irregular rows of conical teeth, similar to those of premaxilla. Barbels well developed, dorsoventrally flattened and tapered distally; variable in length; maxillary barbel usually largest, reaching the base of the pectoral fin, rictal barbel usually shortest, not reaching the base of the pectoral fin ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ). Lower lip with small lateral fleshy lobe located posteromedial to rictal-barbel base. Anterior nostril surrounded by small tubular flap continuous with nasal-barbel base; posterior nostril opening about same size of anterior nostril, with crescent thin flap along anterior margin. Eye without free margin, covered by thin and translucent skin; moderate in size and located dorsolateral, on anterior half of head ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ).
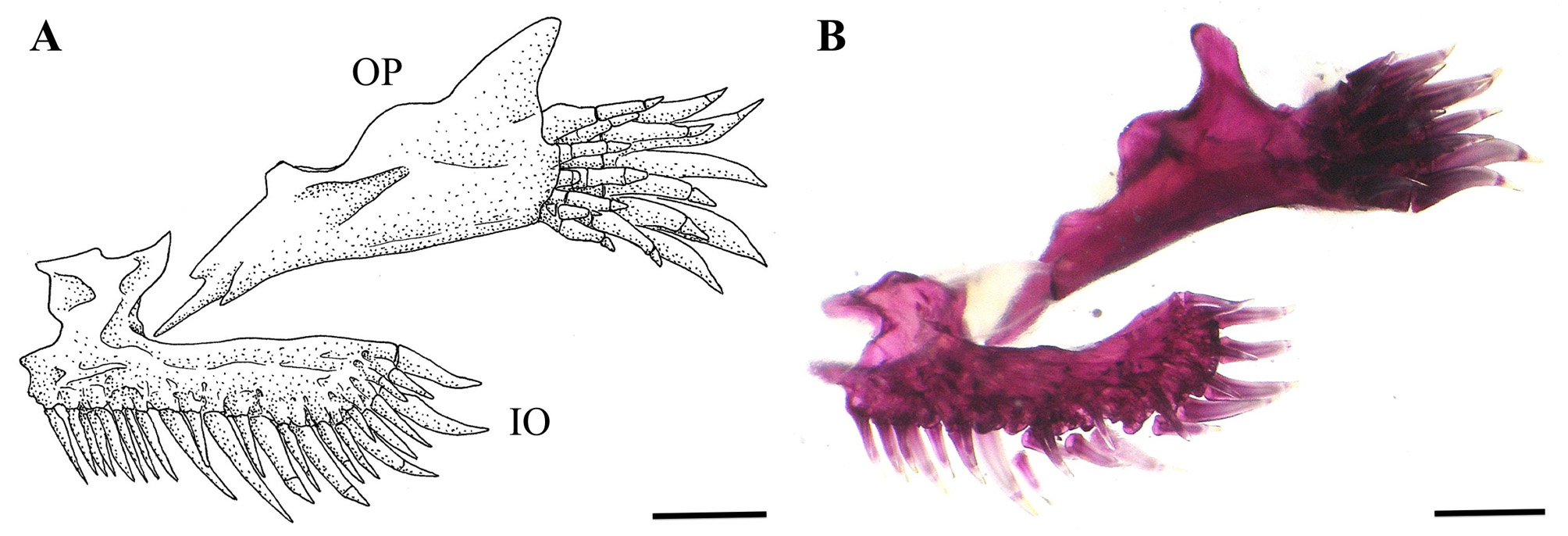
Anterior margin of mesethmoid straight to slightly concave ( Fig. 4 View FIGURE 4 ). Lachrymal-antorbital relatively short and rectangular, compact anteriorly and tubular posteriorly, enclosing most anterior section of infraorbital canal ( Fig. 4 View FIGURE 4 ). Autopalatine with its medial margin slightly concave and its posterolateral process relatively long and pointed ( Fig. 4 View FIGURE 4 ). Sesamoid supraorbital a straight rod, relatively long, without lateral processes ( Fig 4 View FIGURE 4 ). Anterior fontanel small and about oval in shape (its length about 15% of posterior fontanel length); epiphyseal bar entirely osseous, meeting medially; posterior fontanel long, about rectangular in shape, extending from epiphyseal bar to posterior portion of parieto-supraoccipital ( Fig. 4 View FIGURE 4 ). Vomer arrow-head shaped, with lateral processes posterolaterally directed and a long posterior process inserted into anterior process of parasphenoid ( Fig. 4 View FIGURE 4 ). Posterior process of parasphenoid relatively long, extending over anterior portion of basi-exoccipital and laterally bordered by two anterior membranous processes of basi-exoccipital. Metapterygoid laminar and about triangular. Hyomandibula prominent, with a well-developed anterodorsal membranous outgrowth that contacts dorsoposterior tip of metapterygoid. Opercular patch of odontodes oval, with 11–23 (18*) conical odontodes (usually 17) and six to eight (seven*) replacement odontodes; functional odontodes arranged in three to five anteroposterior irregular rows (usually four) ( Fig. 5 View FIGURE 5 ). Interopercular patch of odontodes relatively narrow, posteriorly curved with 27–44 (35*) conical odontodes (usually 33) and 18*–20 replacement odontodes; functional odontodes arranged in three or four lateromedial irregular rows (usually three) ( Fig. 4 View FIGURE 4 ). Odontodes progressively larger and more curved posteriorly in both opercular and interopercular patches ( Fig. 5 View FIGURE 5 ).
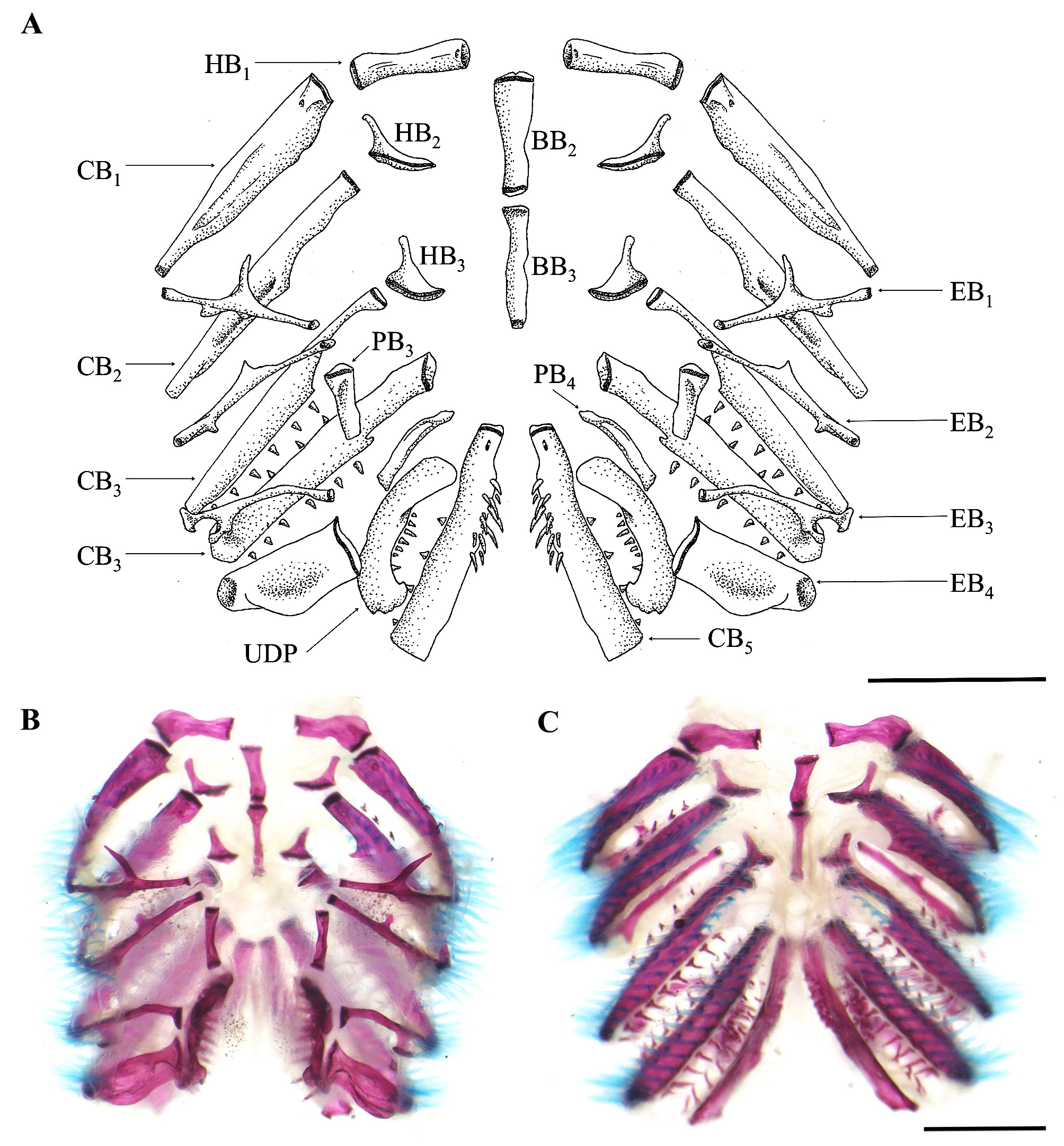
Branchial membrane free from ishtmus, except anteriorly, and supported by seven or eight* branchiostegal rays (usually seven) ( Fig. 6 View FIGURE 6 ). Gill-rakers on first gill arch three to six (usually four) ( Fig. 7 View FIGURE 7 ). Basibranchials 2 and 3 and hypobranchial 1 approximately of same length, about rectangular – in some specimens lateral end of hypobranchial 1 wider and curved posterolaterally ( Fig. 7 View FIGURE 7 ). Hypobranchial 2 boomerang shaped, its ossified portion elongate and anterolaterally oriented; hypobranchial 3 trapezoidal, with ossified portion triangular and anterolaterally oriented ( Fig. 7 View FIGURE 7 ). Ceratobranchial 1 with three to five gill rakers along anterior margin; medial tip wider than lateral tip. Epibranchial 1 with one gill raker along anterior margin, close to articulation with ceratobranchial 1; a prominent anterior uncinate process laterally curved plus a shorter posterior uncinate process, closer to lateral tip. Ceratobranchial 2 with five gill rakers along anterior margin. Epibranchial 2 with one gill raker along anterior margin; short anterior and posterior uncinate processes. Ceratobranchial 3 with five or six gill rakers along anterior margin and eight gill rakers along posterior margin; broad notch at medial portion of posterior margin. Epibranchial 3 with medial portion curved; two gill rakers along posterior margin, close to joint with ceratobranchial 3 and a dorsally curved uncinate process just medial to gill rakers insertion. Ceratobranchial 4 with eight or nine gill rakers along anterior margin and nine along posterior margin. Epibranchial 4 broad with anterior and posterior crests giving a rectangular aspect ( Fig. 7 View FIGURE 7 ), supporting two or three gill rakers along anterior margin. Ceratobranchial 5 with eight or nine gill rakers along anterior margin and 12-17 conical teeth (usually 12), arranged in two irregular rows along anterior portion of medial margin; largest teeth posteromedially placed ( Fig. 7 View FIGURE 7 ). Pharyngobranchials 3 and 4 rectangular ( Fig. 7 View FIGURE 7 ). Upper dentigerous plate with 12-14 conical teeth arranged in two rows; external row restricted to anterior portion, with two to four teeth; internal row complete, along entire medial margin of plate, with 9–13 teeth; teeth of internal row larger than those of external row ( Fig. 7 View FIGURE 7 ).
Levator internus 4 origin on posterior region of dorsal surface of posttemporo-supracleithrum. Extensor tentaculi origin restricted to lateral surface of neurocranium. Insertion of secondary-ventral section of dilatator operculi restricted to dorsal process of opercle. Primary section of dilatator operculi passing medial to levator arcus palatini.
Supraorbital sensory canal continuous with three pores (s1, s2, and s3). Sensory pore s1 medially adjacent to anterior nostril. Sensory pore s3 medial to posterior nostril, at level of its posterior margin. Sensory pore s6 (epiphyseal) paired, close to each other dorsally. Infraorbital sensory canal interrupted in two portions; anterior most portion with sensory pores i1 and i3, laterally adjacent to anterior and posterior nostrils, respectively; posterior most portion connected to supraorbital and otic canals, with sensory pores i10 and i11. Preopercular canal short with single terminal pore anterodorsal to opercular patch of odontodes. Postotic canal with single pterotic branch and associated pore above opercular patch of odontodes. Lateral line canal short with two pores above pectoral-fin base. Sensory pore ll1 ventral to main lateral line canal and ll2 terminus of main lateral line canal.
Total vertebrae 36–37* (usually 36) (precaudal vertebrae four, caudal vertebrae 32–33). Ribs 12–14*, usually 13. First hemal spine on vertebra 15* or 16.
Pectoral fin with i, 7* or 8 rays, usually i,7; first ray extended beyond fin margin as a long filament; filament length about equal to head length; posterior margin straight to convex; anterior portion of fin base covered by branchial membrane ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ). Cleithrum with broad foramina under scapulocoracoid and adjacent to hypocoracoid portion; coracoid bridge variably long and usually pointed ( Fig. 6B View FIGURE 6 ). Pelvic fin with i,4 rays and one pelvic splint; first ray shortest and third ray longest; origin of fin anterior to dorsal-fin origin, at posterior half of body (between free vertebrae 17 or 18); distal margin usually not reaching urogenital opening. Basypterigium with two relatively long anterior processes, approximately of same length and an anterior symphyseal process of about 15% of anterior processes length ( Fig. 6C View FIGURE 6 ); a posterior symphyseal process variably present; bases of pelvic fins in contact, not widely separated ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ). Dorsal fin with three* or four procurrent and ii, 6 or 7* principal rays, usually ii, 7; second branched ray usually longest; fin about rectangular in shape in lateral view, with posterior margin truncated; fin origin located anterior to vertical through anus, usually over or slightly posterior to distal portion of pelvic fin; basal and anterior portions of fin extensively covered by thick integument, with anterior most two or three unsegmented (procurrent) fin rays hardly visible externally ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ). Dorsal-fin pterygiophores inserted between neural spines of vertebrae 18–23 or 19–24*. Anal and urogenital openings located approximately midway between pelvic-fin insertion and anal-fin origin. Anal fin with three* or four procurrent and ii,5*–6 principal rays, usually ii, 5; second branched ray usually longest; fin slightly shorter than dorsal fin, similar in shape to dorsal fin; origin of fin located at a vertical between bases of fourth and fifth branched dorsal-fin rays; basal and anterior portions of fin extensively covered by thick integument, with anterior most three unsegmented (procurrent) fin rays hardly visible externally ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ). Anal fin pterygiophores inserted between hemal spines of vertebrae 20–24 or 22–26*. Caudal skeleton with three plates, PH +1+2, 3, 4+5; epural absent; neural spine of preural centrum 1 complete with anterior and posterior processes at base of spine variably present ( Fig. 8 View FIGURE 8 ). Caudal fin with i, 10–12, i principal rays, usually i, 11, i*, and 13–18 (16*) dorsal procurrent rays, usually 15, and 12–15 (13*) ventral procurrent rays, usually 14, originating posterior to neural and hemal spines of vertebra PU7 and PU7 or PU6*, respectively; distal margin of caudal fin rounded, mostly in juvenile specimens (less than 65.0 mm SL), to truncate, mostly in late juvenile and adult specimens (more than 70.0 mm SL) ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ).
Color in alcohol. Body light brown, uniform or with a black lateral band or small (less than eye diameter) dark brown spots on dorsal surface and sides; dorsal surface darker, ventral surface paler. Fins translucent or light brown, darker basally. Caudal peduncle with a longitudinally elongated light brown spot ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ).
From a subsample of 38 specimens, ranging from 27.3 to 119.8 mm SL, a total of 20 (52.6%) specimens showed the herein called coloration pattern or phenotype I ( i.e., body light brown with a black lateral band; Figs. 2A–C, E View FIGURE 2 ); 12 (31.6%) specimens showed the coloration pattern II ( i.e., body light brown uniform, without a black lateral band or spots); and six (15.8%) specimens showed the coloration pattern III [ i.e., body light brown without a black lateral band but with several small (less than eye diameter) dark brown spots on dorsal surface and sides, more evident in the posterior half of the body]. In small specimens (less than 65.0 mm SL; n = 18) the coloration pattern I was dominant (83.3%; i.e., 15 specimens), while no specimens with the coloration pattern II where recorded; only 16.7% (three specimens) showed the coloration pattern II. In medium-sized specimens (65.0–100.0 mm SL, n = 14), the coloration pattern II was the least frequent (21.4%; i.e., three specimens); whereas the coloration patterns I and III were displayed by 5 (35.7%) and 6 (42.9%) specimens, respectively. On the other hand, the color pattern III was displayed in all large specimens (more than 100.0 mm SL; n = 6). Such results could suggest an ontogenetic change in the coloration pattern from state I, predominant in small (juvenile) specimens, to state III, predominant in large (adult) specimens, condition that has already been reported in other congeneric species [see da Silva et al. (2010) and Nascimiento et al. (2017)].
Color in life. Body brown, light brown, yellowish or light orange, darker on dorsal surface, and paler ventrally, usually with a continuous black lateral band (of variable thickness), extending from opercle to caudal-fin base. Lateral band absent in some specimens, having a coloration pattern more or less uniform (usually light brown or yellowish) with or without small (less than eye diameter) dark brown spots on dorsal surface and sides. Paired, dorsal and anal fins light brown, yellowish (usually darker at base) or translucent; caudal fin brown or yellowish, usually darker than other fins. Caudal peduncle with a longitudinally elongated light brown to black spot ( Fig. 3 View FIGURE 3 ).
Geographic distribution. Trichomycterus striatus was originally described from the Cana River (Tuyra River basin) in eastern Panama, later being recorded in most of the main river basins in both the Pacific and Atlantic versants of the country ( Behre 1928, Hildebrand 1938, Loftin 1965, Smith & Bermingham 2005). This species has also been reported from Costa Rica ( Bussing 1967, 1998, Angulo et al. 2013), along the Pacific slope from the Térraba and Coto River basins, making it the trichomycterid species with the northern-most distribution. Specimens collected in Uvita, Puntarenas, Costa Rica (DZSJRP 21188; Fig. 3 View FIGURE 3 ) represents the first documented record of the species in the Pirrís River basin, sensu Angulo et al. (2015), which expands, slightly, the northern geographic limit of the species (genus and family) in lower Central American waters ( Fig. 9 View FIGURE 9 ).
On the other hand, there are numerous records of occurrence of T. striatus in Colombian waters [ e.g., Eigenmann (1918), Mojica-C. (1999), Maldonado-Ocampo et al. (2005, 2008, 2012), Ortega-Lara et al. (2006), Villa-Navarro et al. (2006), Jaramillo-Villa et al. (2008), Castellanos-Morales & Galvis (2012), among others]; however, the examination and direct comparison of specimens collected from both the Atrato and Magdalena River basins identified as T. striatus with specimens from Panama and Costa Rica, have revealed some morphological differences, raising doubts on the actual occurrence of the species in Colombia. The resolution of the taxonomic status of the Colombian populations of T. striatus is currently under study by one of the authors (CD), thus we herein opt to defer the inclusion of these previous records of T. striatus from Colombian river basins.
Ecological notes. Trichomycterus striatus inhabits forest brooks and creeks, of low to moderate current velocity, with bottoms consisting mainly of sand or small to medium sized rocks, with periphytic and/or submerged vegetation usually present ( Bussing 1998). In Costa Rica, T. striatus has been reported at elevations ranging from 20 to 660 m ( Bussing 1998, Angulo et al. 2013), reaching up to 1220 m in Panama ( Behre 1928, Bussing 1998). Kramer & Bryant (1995) determined that in the Chagres River basin, Panama, this species fed almost exclusively on benthic aquatic invertebrates, mainly insect larvae. Bussing (1998) reported a maximum length of 120 mm TL, whereas de Pinna & Wosiacki (2003) reported a maximum length of 84 mm TL. Specimens collected in Costa Rica (DZSJRP 21188) and Panama (UCR 434-4), reaching up to 135 mm TL, represents a new size record for the species.
Common and vernacular names. Pencil catfish (English; Bussing 1998); Babosa, Barbudito, Barbudo, Laucha, Pez gato lápiz (Spanish; Angulo 2013).
Remarks. Meek & Hildebrand (1913) cited only the holotype (FMNH 7579) of their newly described Pygidium striatum , collected in the Cana River (Tuyra River basin), eastern Panama. However, it is clear that the original description accounted for several specimens as indicated from the ranges provided for head length, body depth, head width, interorbital width, eye diameter, snout length, first pectoral-fin ray length, caudal peduncle length, and caudal peduncle depth, as well as variation of caudal-fin shape and body coloration (where even largest specimens are cited, in relation to the most numerous presence of spots on the lower region of the side of the body in these specimens). Later, Meek & Hildebrand (1916), in a redescription of the species, explicitly indicated the availability of 32 specimens ranging from 40 to 90 mm, all obtained from the same locality, giving a more accurate description of the collecting site (small creek at Cana in the upper Río Tuyra Basin). Eigenmann (1918) also cited the presence of several types examined by him in his redescription of the species. Ibarra & Stewart (1987) in their catalog of types at FMNH listed 18 paratypes (associated catalog numbers were not indicated) from the same locality of the holotype. Ferraris & Vari (1992) also listed paratypes at USNM, distributed in two lots (78374, 78375) with four and 11 specimens, respectively. However, these authors remarked about the probable type status of these specimens, given that the original description only cited the holotype, but was based in more than one specimen. The type status of these presumed paratypes (both at FMNH and USNM) is uncertain, given that the International code of zoological nomenclature ( ICZN, 1999) does not offer a comparable situation. In the meanwhile, these paratypes could be still treated as such, following the Article 72.4.1.1 of the code: “For a nominal species or subspecies established before 2000, any evidence, published or unpublished, may be taken into account to determine what specimens constitute the type series”.
Behre (1928) described Pygidium septentrionale from 12 specimens, collected in the Salao Creek ( Chiriquí River basin), western Panama. This species was diagnosed from T. striatus by having a shorter and broader head, a more anteriorly placed anal fin and a darker body, covered by several dark brown spots. Hildebrand (1938) and Loftin (1965) recorded T. striatus in several localities from both the Pacific and Atlantic drainages of Panama, but notably, they did not record specimens of T. septentrionale in their collections. Later, Bussing (1967) assigned two specimens from Costa Rica to T. striatus (being the first occurrence record of the genus in this country), comparing them with four specimens from Panama collected by Loftin in 1965. Bussing (1967) also noted a high degree of intraspecific variation in some morphometric characters ( e.g. eye diameter and barbels length) and in the coloration pattern, based on the material examined by him and the original description of T. striatus , questioning the validity of T. septentrionale . Finally, Bussing (1998) proposed T. septentrionale as a junior synonym of T. striatus , based on the examination of additional specimens from Costa Rica and Panama (10 and 15, respectively), given the overlapped broad variation in the characters used by Behre to recognize T. septentrionale from T. striatus . The data gathered in this study supports the conclusions of Bussing (1998), recognizing T. striatus as the sole representative of the genus in lower Central America.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Trichomycterus striatus ( Meek & Hildebrand, 1913 )
| Angulo, Arturo, Donascimiento, Carlos, Lasso-Alcalá, Oscar M., Farah-Pérez, Aldo, Langeani, Francisco & Mcmahan, Caleb D. 2018 |
Pygidium striatum
| Meek & Hildebrand, 1913 : 78 |
| Meek & Hildebrand, 1916 : 266 |
| Eigenmann, 1918 : 221 |
| Eigenmann, 1922 : 60 |
Trichomycterus striatus
| Bussing, 1987 : 110 |
| Ibarra & Stewart, 1987 : 12 |
| Burgess, 1989 : 323 |
| Ferraris & Vari 1992 : 42 |
| Kramer & Bryant, 1995 : 129 |
| Bussing, 1998 : 159 |
| Pinna & Wosiacki, 2003 : 285 |
| Smith & Bermingham, 2005 : 1840 |
| Ferraris, 2007 : 424 |
Pygidium septentrionale
| Behre, 1928 : 309 |
| Henn, 1928 : 81 |
| Ibarra & Stewart, 1987 : 73 |
Trichomycterus septentrionale
| Burgess, 1989 : 323 |