Gromphas dichroa, BLANCHARD, 1846
|
publication ID |
https://doi.org/10.5252/zoosystema2024v46a2 |
|
publication LSID |
lsid:zoobank.org:pub:4B49C1D9-1196-4942-969F-2E923B1FC12C |
|
DOI |
https://doi.org/10.5281/zenodo.10668043 |
|
persistent identifier |
https://treatment.plazi.org/id/03C5B216-FFFE-FFA6-DDED-FD8C6E9DFE03 |
|
treatment provided by |
Plazi |
|
scientific name |
Gromphas dichroa |
| status |
|
NEW RELEVANT RECORDS FOR THE VANISHED GROMPHAS DICHROA BLANCHARD, 1846 View in CoL
Cupello & Vaz-de-Mello (2013, 2015) discussed the rarity of G. dichroa in collections. Only 9 males and 9 females were known to us apart from the missing holotype 3, the lost six specimens studied by Barattini & Sáenz (1961, 1964), and the two equally lost specimens mentioned by Burmeister (1874). No individuals were known to have been caught since 1947 despite intensive collecting efforts across the species’ range from southern Paraguay to the Argentinian province of Buenos Aires4. Even Antonio Martínez (1922- 1993), who collected and studied the Argentinian dung beetle fauna so extensively through the mid- and late-20th century, never collected a G. dichroa or obtained a specimen caught by others after the 1940s. The same is reported by Federico Ocampo (personal communication, 21 June 2021), who has done fieldwork more recently in areas within the historical range of G. dichroa , including Uruguay and Buenos Aires. For these reasons, I started to wonder whether G. dichroa may have become extinct.
Since the publication of Cupello & Vaz-de-Mello’s revision, I had the opportunity to visit several other collections in Europe and the Americas, including all the main ones in southern Brazil and Paraguay, and in just two of them did I find additional specimens of G. dichroa . One is the Brussels museum ( RBINS), which houses a single specimen. As can be seen in Figure 11B, C View FIG , this specimen shows the bicoloured colouration pattern of the species (similar to fig. 14a of Cupello & Vaz-de-Mello 2013) and bears only two labels, neither informing where and when the specimen was collected. But this certainly happened before 1935, for the accession number ‘10.640’ stated on one of its labels refers to material acquired on 5th October that year by the RBINS from the Belgian coleopterist Joseph Jean Edouard Gillet (1865-1937) (Alain Drumont, Coleoptera curator at RBINS, personal communication, 16th March 2020). This same specimen was mentioned five years later by Janssens (1940) in his study of the RBINS phanaeines, a work overlooked by Cupello & Vaz-de-Mello. Regrettably, I did not have time to sex the specimen during my stay in Brussels, nor did Janssens mention it in his paper.
The second collection where I found additional specimens of G. dichroa is that of the Museu Anchieta de Ciências Naturais, Colégio Anchieta, Porto Alegre, Brazil ( MGAP). There, I found one male and three females collected between 1928 and 1954 in the Brazilian states of Rio Grande do Sul and Santa Catarina ( Fig. 11D View FIG ). These data represent the most recent record for the species (seven years later than the previous most recent record from 1947) and the first report for Santa Catarina; furthermore, the Rio Grande do Sul specimen caught in Nova Petrópolis represents a new municipality record in the state. These two new records also confirm that the species at least used to occupy areas not only in the Uruguayan Savanna ecoregion (also known as the Pampas), from where most of the specimens are known, but also in the bordering southern fringe of the Atlantic Forest; whether they were caught in the rainforests themselves, in transitional areas, or in deforested farmlands is unclear. The four MGAP specimens encompass much of the colour variation shown by G. dichroa : both Rio Grande do Sul females have the typical bicoloured pattern of the species, with a shiny red head and pronotum and blue elytra, whereas the Itapiranga individuals show either the body entirely green (the male) or its head and pronotum red at centre and green over the lateral edges and its elytra uniformly green (the female). As discussed and illustrated by Cupello & Vaz-de-Mello (2013, 2015), this whole variation can occur within a single population, and the bicoloured pattern evolved in parallel in at least two other sympatric lineages of dung beetles, namely Bolbites onitoides Harold, 1868 ( Cupello et al. 2021) and the clade formed by Canthon lividus Blanchard, 1846 and C. auricollis Redtenbacher, 1868 ( Vaz-de-Mello & Cupello 2018b).
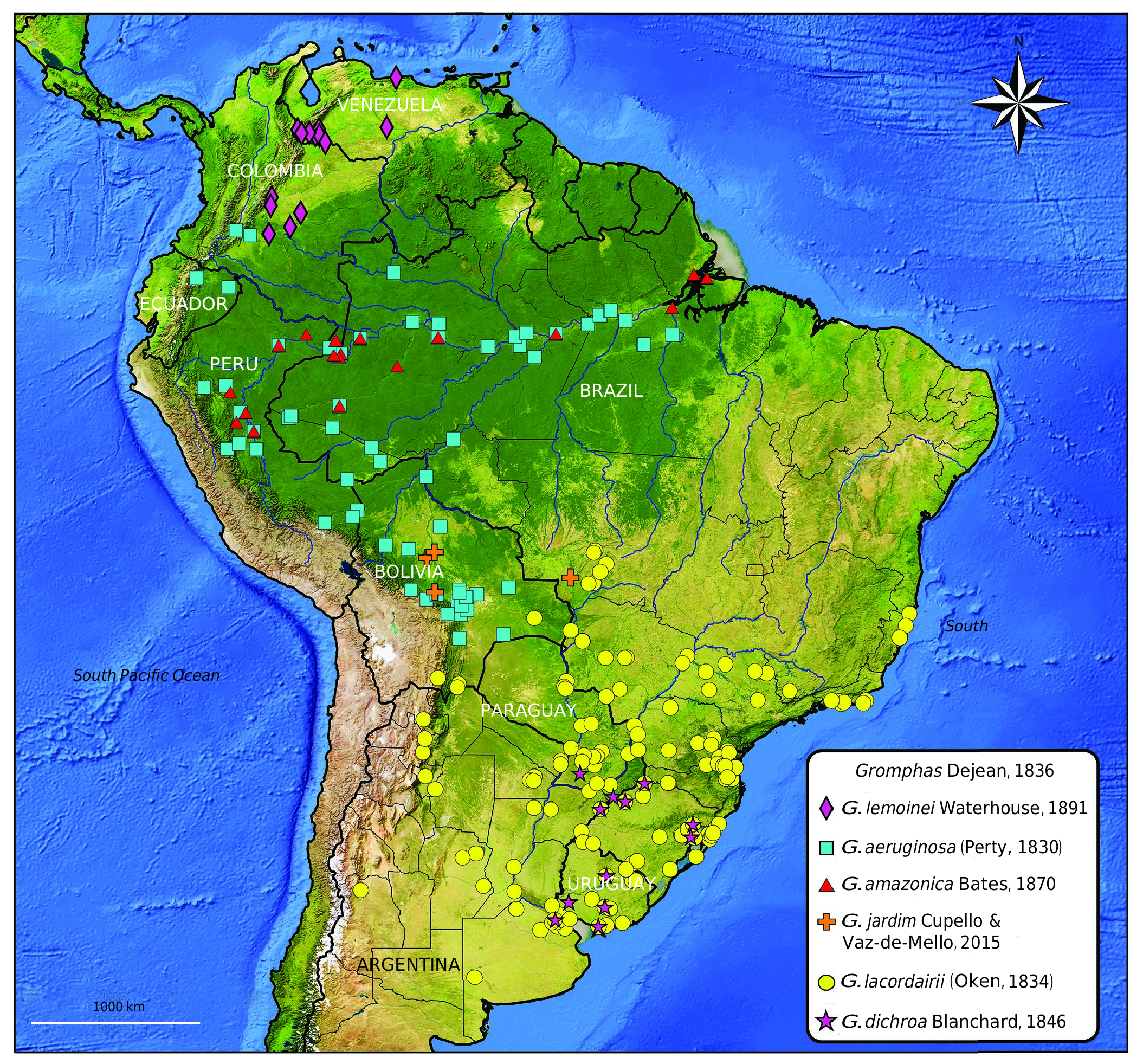
The MGAP is only one of the two museums in Porto Alegre, the capital city of the state of Rio Grande do Sul and which is (or was) part of the range of G. dichroa , the other being the Museu de Ciências Naturais da Fundação Zoobotânica do Rio Grande do Sul ( MCNZ). While the MGAP collection grew rapidly during the first half of the 20th century, it has been mostly inert since the death of its founder, Father Pio Buck (1883-1972), and houses few specimens collected after the 1950s. It is not surprising, therefore, that no G. dichroa collected after that decade is held there. On the other hand, the MCNZ was founded as a separate museum only in 1954 ( Nedel 2005) and owns scarabaeine material collected mostly thereafter. Its dung beetle collection is particularly rich and includes material collected all over Rio Grande do Sul, including several rare species native to the state and other regions of Brazil (personal observation in 2018). Were G. dichroa still living in that region, it would be expected that at least some specimens would be housed in the MCNZ. But this is not the case. Why? This becomes even more intriguing because Gromphas lacordairii , a closely related species ( Cupello & Vaz-de-Mello 2015), is abundant both in the MGAP and in the MCZN and continues to be commonly caught throughout the region where G. dichroa used to be found, including specific localities such as Porto Alegre (last G. dichroa from 1928; last G. lacordairii from 1993) and the greater Buenos Aires area (1935 and 2006, respectively) ( Figs 12-14 View FIG View FIG View FIG ; Table 1 View TABLE ).
But despite its decades-long disappearance and my suspicion of possible extinction, I concede that no formal assessment of the conservation status of G. dichroa is currently possible under the criteria set out by the IUCN Species Survival Commission (2012). At least the historical extent of its geographical occurrence, beyond 600000 km 2, is way too large for any of the IUCN threatened categories (maximum of 20 000 km2 for vulnerable), and nothing in terms of the species’ population sizes and trends, habitat preferences, current area of occupancy, and life habits is available for a proper judgement. Formally classifying the species as ‘extinct’, in particular, would be, for the moment, too precipitate as G. dichroa does not match the IUCN’s requirement of having been the special target of exhaustive surveys in all places where it is known to have been historically present as well as others where it might be expected to occur. Until these are performed using all the collecting techniques available for dung beetles (not only pitfall traps baited with excrement; see below), the species is better classified as ‘data deficient’. Critical localities are those where even G. lacordairii has not been recorded over the last decades, for this denotes a general lack of collection effort. These include, in Argentina, Santo Tomé (last G. dichroa from 1928; last G. lacordairii from 1926) and Santa Maria (last G. dichroa from 1947, no G. lacordairii ), and, in Brazil, Cerro Largo (1941 and 1949, respectively), Nova Petrópolis (both species last recorded in 1928), and Itapiranga ( idem in 1954). Also worthy of attention are the Campanha Gaúcha and Serras de Sudeste regions of the Brazilian state of Rio Grande do Sul. Although G. dichroa has never been recorded from there, these regions are apparently within its range and still encompass vast extensions of relatively pristine areas of the Pampas, particularly around the nature preserve Área de Proteção Ambiental Ibirapuitã, on the border of Brazil and Uruguay (see Souza et al. 2020 ’s map). Should all these future collections be performed and, yet, G. dichroa continue failing to appear, then we can finally ‒ and unfortunately ‒ deem the species extinct. Nevertheless, if any of the localities does retrieve the species, a study of the re-discovered populations will enable a formal assignment to one of the IUCN categories. Another dung beetle species occupying much the same range as G. dichroa , Anisocanthon pygmaeus ( Gillet, 1911) , and which has, too, vanished since the 1950s, has also been classed as data deficient by Vaz-de-Mello et al. (2014). My treatment thus follows the established convention.
This prudence is indeed justified. A number of Brazilian dung beetles that, like G. dichroa , had vanished during the second half of the 20th century have been recently re-discovered either in new populations or in the same area where they were previously known. A representative of the latter case is Paracryptocanthon borgmeieri (Vulcano, Pereira & Martinez, 1976) , which until recently was known from just seven females collected in the 1960s and 1970s and which was rediscovered in 2013 through the collection of a large series of specimens of both sexes at the type locality ( Pacheco & Vaz-de-Mello 2017). Its almost 40-year disappearance is particularly remarkable because the species’ entire range lies in the heart of the heavily collected city of Rio de Janeiro. In turn, a case of a vanished species that was later rediscovered living far from its previously known range is Sulcophanaeus rhadamanthus (Harold, 1875) . After being known for decades from a few old specimens collected in the Serra da Mantiqueira and Serra dos Órgãos mountain ranges in southeastern Brazil ( Edmonds 2000; the author does not provide precise label data of the specimens he examined, but it is likely that they were all collected before the 1950s), this species was recently rediscovered living about 1000 km farther south in the municipality of Santa Maria in the Rio Grande do Sul state ( da Silva et al. 2011, 2012 a, 2013) and, subsequently, a little farther north in the municipality of Anitápolis, Santa Catarina state ( Simões-Clivatti & Hernández 2022). These rediscoveries of long-lost dung beetles are, in fact, not limited to the Brazilian fauna, as recently shown by Deschodt et al. (2021) in Madagascar and Hielkema (2023) in Suriname.
But, if not extinction, what else could explain the widespread disappearance of G. dichroa whereas G. lacordairii continues to be abundant? Part of the explanation may be that the populations of G. dichroa have always been naturally small and sparse. The number of specimens collected before the 1950s, for example, was already much smaller than that of G. lacordairii ( Fig. 14 View FIG ). Kohlmann (1991) and Price & May (2009) have shown that pairs of sympatric phanaeine species with similar body size like the dichroa / lacordairii pair usually differ drastically in their local relative abundance, from a ratio of c. 3 individuals per 7 to 1/100. A similar proportion was found by Cupello & Vaz-de-Mello (2018) for the deltochilines Sylvicanthon seag Cupello & Vaz-de-Mello, 2018 and S. securus (Schmidt, 1920) in northern Amazonia, although the exact figures vary from place to place. More impressive still, Feer (2000) observed the same phenomenon across an entire Scarabaeinae community in French Guiana. This persistent pattern may be the result of the competitive exclusion principle ( Hardin 1960). One of the competitors, being more efficient in a given context, multiplies and pushes the other to lower abundances or even complete local extinction. This in itself may already explain why G. dichroa has always been rarer: it is less competitive than its close ally and always sympatric G. lacordairii .
And there may be more to this. In evolutionary time, this continuous pressure from competition may eventually lead the rarer, less competitive species to niche specialisation through ecological character displacement ( Brown & Wilson 1956; Pfennig & Pfennig 2009). Perhaps the competition with the presumably more efficient G. lacordairii drove G. dichroa to stenotopy. And if, in this specialisation, G. dichroa departed from the usual coprophagous, non-inquiline behaviour of most dung beetles, including G. lacordairii , maybe becoming, for example, mycetophagous or an inquiline of insect or vertebrate nests, this would explain why it has not been attracted to regular dung traps. Edmonds (2000) indeed suggested that S. rhadamanthus , with a body size similar to its sympatric and possibly sister species S. menelas (Castelnau, 1840) , was rarer for possibly having such an idiosyncratic biology, and Cupello & Vaz-de-Mello (2018) made a similar suggestion to explain why S. securus has been collected so less frequently than S. seag . Perhaps this is also the case with G. dichroa and G. lacordairii . Indicating that G. lacordairii is indeed more eurytopic and competitive than G. dichroa is that it has a much broader geographical range and inhabits a more diverse set of biomes than its rarer relative ( Fig. 5 View FIG ; Cupello & Vaz-de-Mello 2013, 2015).
But low abundance and stenotopy in themselves, while explaining rarity in the field and collections, would still leave unanswered the question of why G. dichroa has not been collected since the 1950s as these factors did not prevent the species from being collected before. An explanation could be that early-20th-century collectors applied collection procedures more suitable for the capture of G. dichroa than modern collectors do, such as the search for specimens in ant nests. Even though, judging from its morphology, G. dichroa does not seem to be a myrmecophilous species, we know, for instance, that the likely collector of the MGAP series, Father Pio Buck, used to search for beetles in ant nests (e.g., the type series of Ateuchus myrmecophilus ( Boucomont, 1935) was collected by him in an Acromyrmex lobicornis (Emery, 1888) nest; see Boucomont 1935). It may also be that G. dichroa is a saprophagous species attracted to decaying fruits or fungi, and the current preferred usage of mammalian excrement and carrion as bait (e.g., Morelli et al. 2002; da Silva 2017; da Silva et al. 2008, 2009; da Silva & Di Mare 2012; Canziani & González-Vainer 2022; but see da Silva 2011; da Silva et al. 2012a, b, 2013; da Silva & Bogoni 2014) may have been inefficient in attracting the species. In sum, it may be that the disappearance of G. dichroa since the 1950s is simply the consequence of a peculiar biology (this itself a result of the competition with the more ecologically aggressive G. lacordairii ) that keeps population densities low and spatial presence limited, coupled with the fact that modern collectors are not looking in the right places or applying the right collecting techniques that earlier naturalists, by chance or design, employed.
But having now examined all the difficulties inherent in the idea and explored its alternatives, we can at least recognise the plausibility of my initial hypothesis, extinction. If this is indeed the case, causes that could have driven G. dichroa to extinction are certainly multifactorial and likely included at least two of the elements discussed above, competition with sympatric species such as G. lacordairii and potential strict ecological specialisations. But chief among the factors is likely the negative impact of the ongoing anthropogenic conversion of habitats in southern and central South America. It may be, as briefly explored by Cupello & Vaz-de-Mello (2013), that the intense human pressure converting the forests and natural grasslands from Paraguay and southern Brazil to Argentina and Uruguay into urban space, pastures, and agricultural fields over the second half of the 20th century, particularly from the 1960s Green Revolution onwards ( Roesch et al. 2009; Souza et al. 2020; Ribeiro et al. 2021), impacted the populations of the putatively stenotopic G. dichroa , and this drove a specialised and already fragile species to extinction or at least to a drastic reduction in abundance and range. Gromphas lacordairii , in turn, a more eurytopic and, thus, adaptable species, has not only resisted but continued to flourish. For the time being, however, the fate of G. dichroa will remain uncertain. Future fieldwork may, after all, rediscover the species living in some of the region’s last areas of natural grasslands, wetlands, river sandbanks, floodplains, shrublands, and gallery forests, which, based on its widespread presence in the northern Pampas and the biology of congeneric species, are the candidate preferred habitats of G. dichroa . This would be an exciting discovery, finally allowing us to better understand the biology and conservation status of this intriguing and beautiful species.
MATERIAL EXAMINED. — Gromphas dichroa : Brazil. Rio Grande do Sul • 1♀; Cerro Largo; III.1941; no collector (but likely Pio Buck leg.); MGAP • 1♀; Nova Petrópolis; I.1928; no collector (but likely Pio Buck leg.); MGAP. — Santa Catarina • 1 ♀; Itapiranga; XI.1934; no collector (but likely Pio Buck leg.); MGAP • 1 ♂; Itapiranga; X.1954; no collector (but likely Pio Buck leg.); MGAP. No data: • 1 unsexed specimen; ex J. J. Gillet collection; RBINS .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Scarabaeinae |
|
Genus |