Smilax Laporte, 1835
|
publication ID |
https://doi.org/10.11646/zootaxa.4162.2.5 |
|
publication LSID |
lsid:zoobank.org:pub:1F76AD06-AA68-42C7-BBAF-DA6CF276E332 |
|
DOI |
https://doi.org/10.5281/zenodo.6089849 |
|
persistent identifier |
https://treatment.plazi.org/id/03C78788-A915-2E65-FF46-0DEBDF52A3EC |
|
treatment provided by |
Plazi |
|
scientific name |
Smilax Laporte, 1835 |
| status |
|
Smilax Laporte, 1835 View in CoL
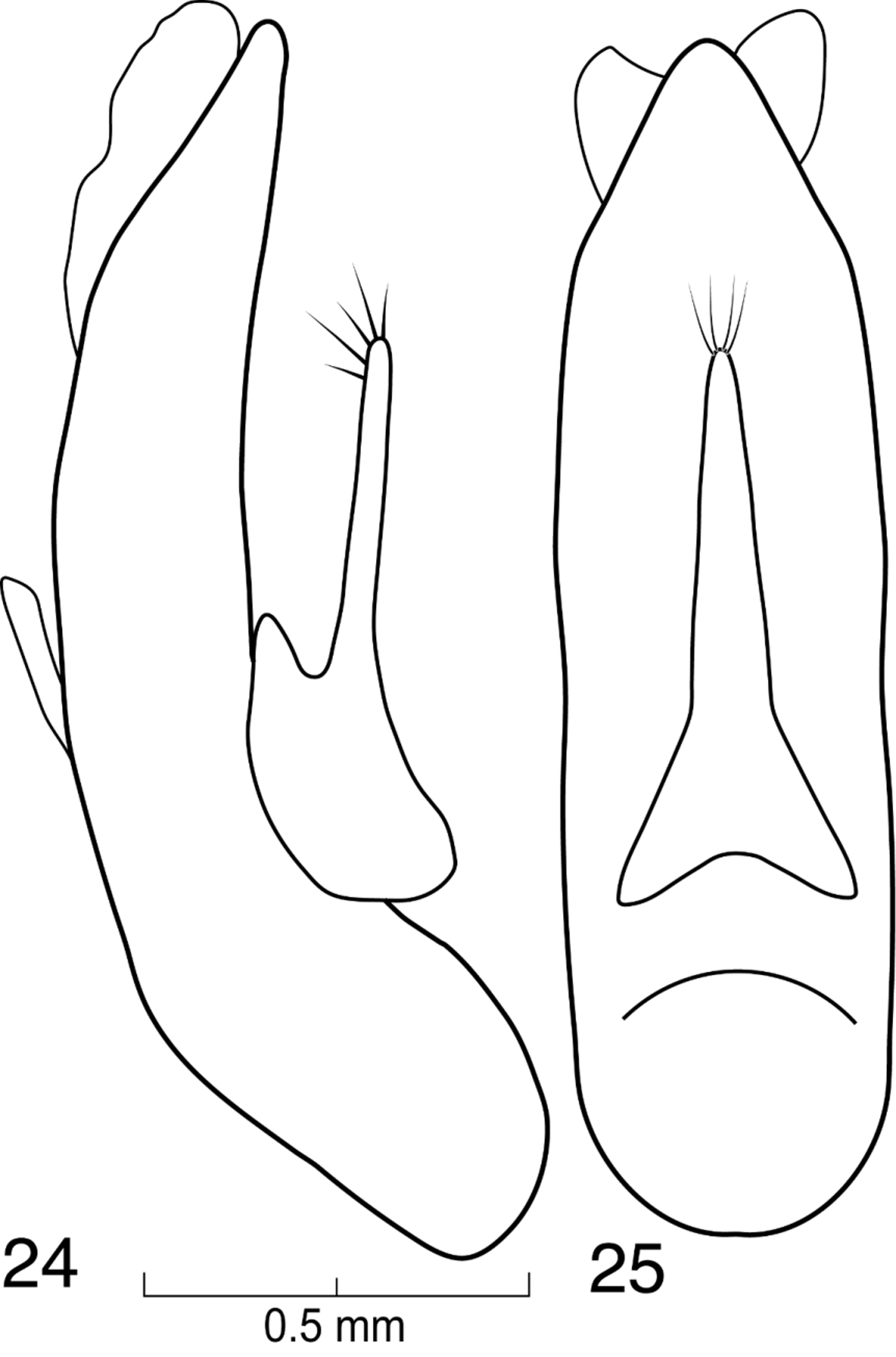
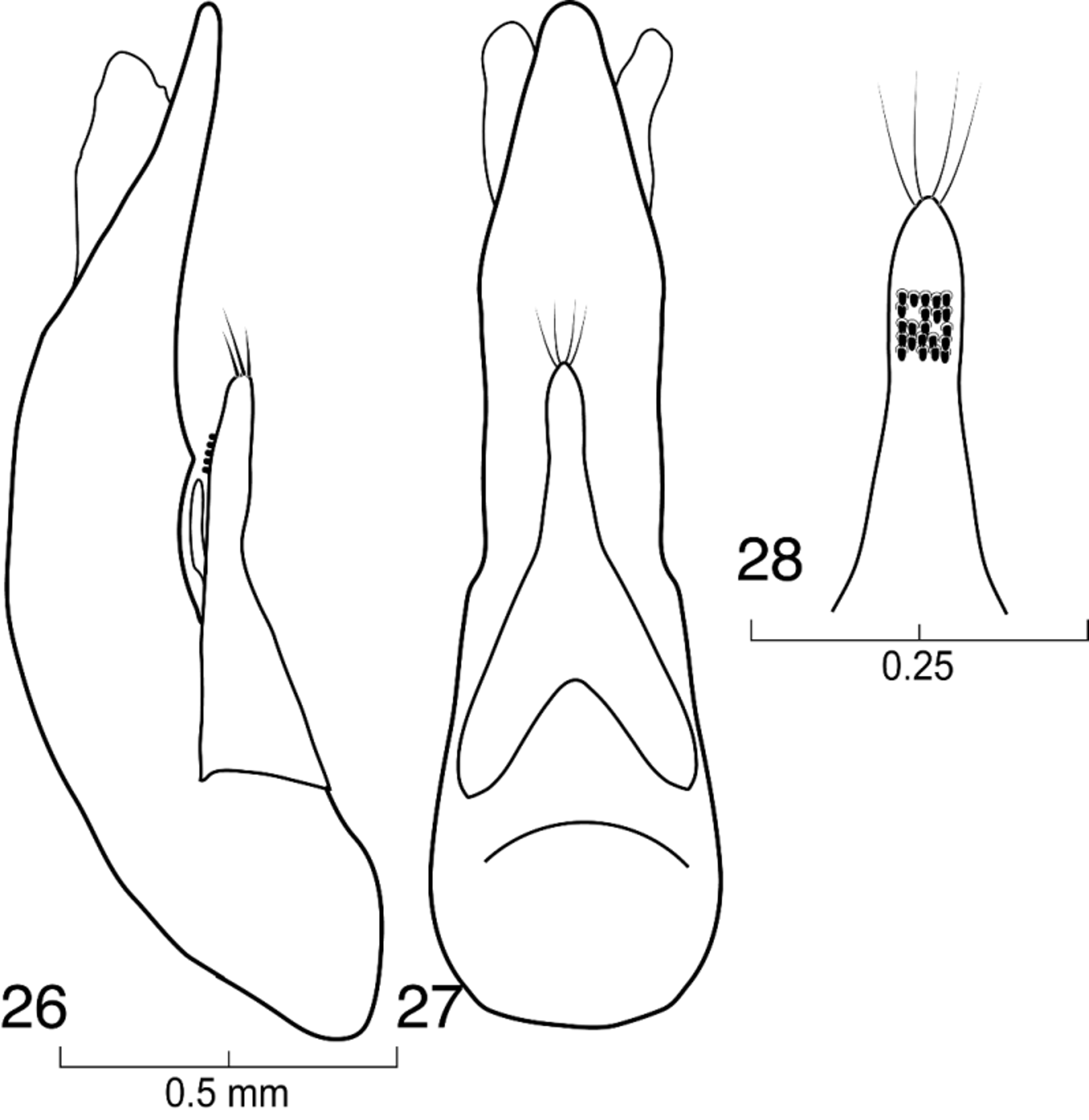
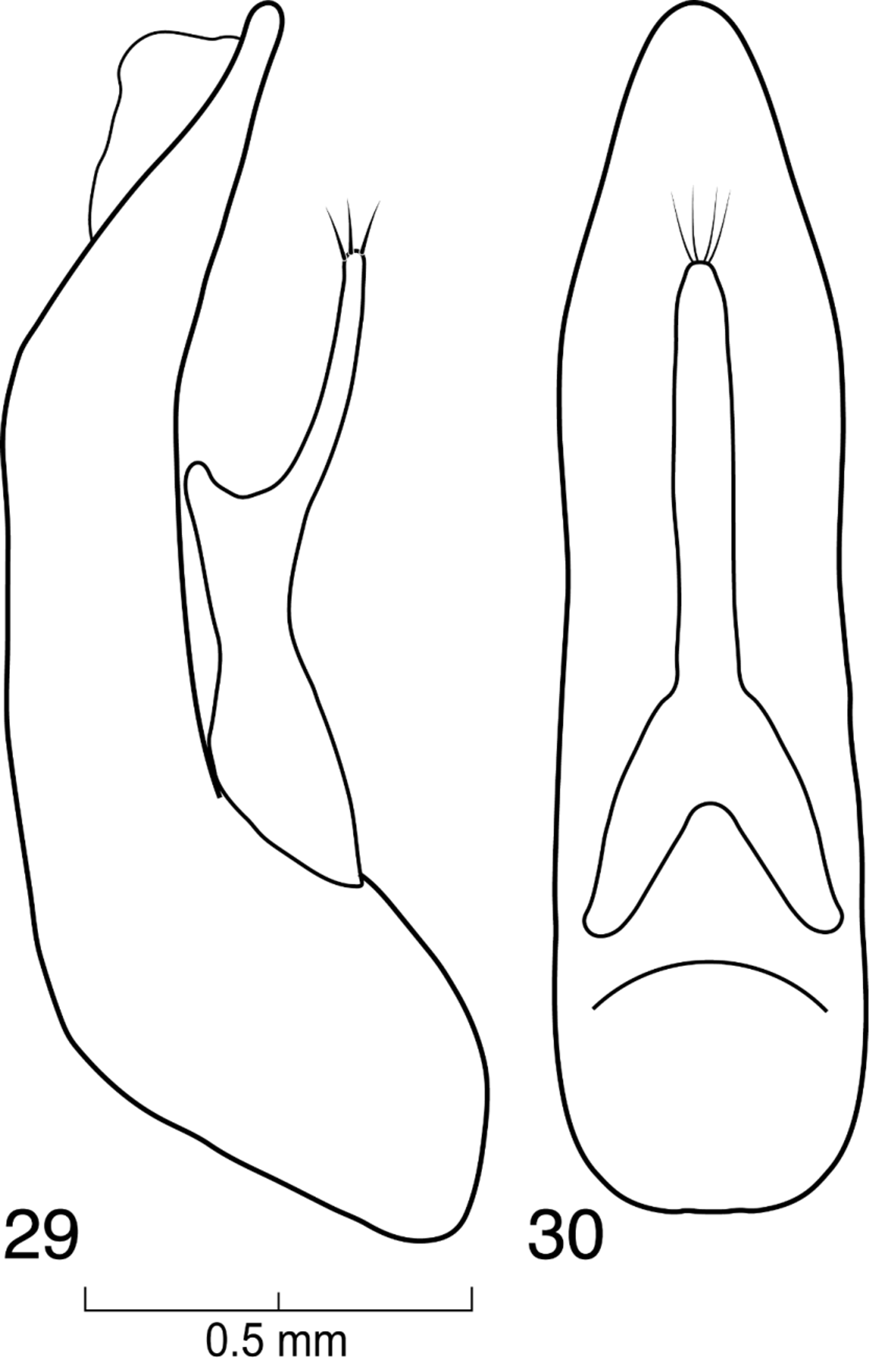
( Figs. 1–34 View FIGURES 1 – 2 View FIGURES 3 – 4 View FIGURES 5 – 8 View FIGURES 9 – 12 View FIGURES 13 – 16 View FIGURES 17 – 23 View FIGURES 24 – 25 View FIGURES 26 – 28 View FIGURES 29 – 30 )
Cordylaspis Nordmann, 1837: 17 View in CoL [Type species: C. tuberculatus Nordmann View in CoL , fixed by monotypy]; Blackwelder 1952: 107.
Type species. Smilax americana Laporte, 1835 , fixed by monotypy.
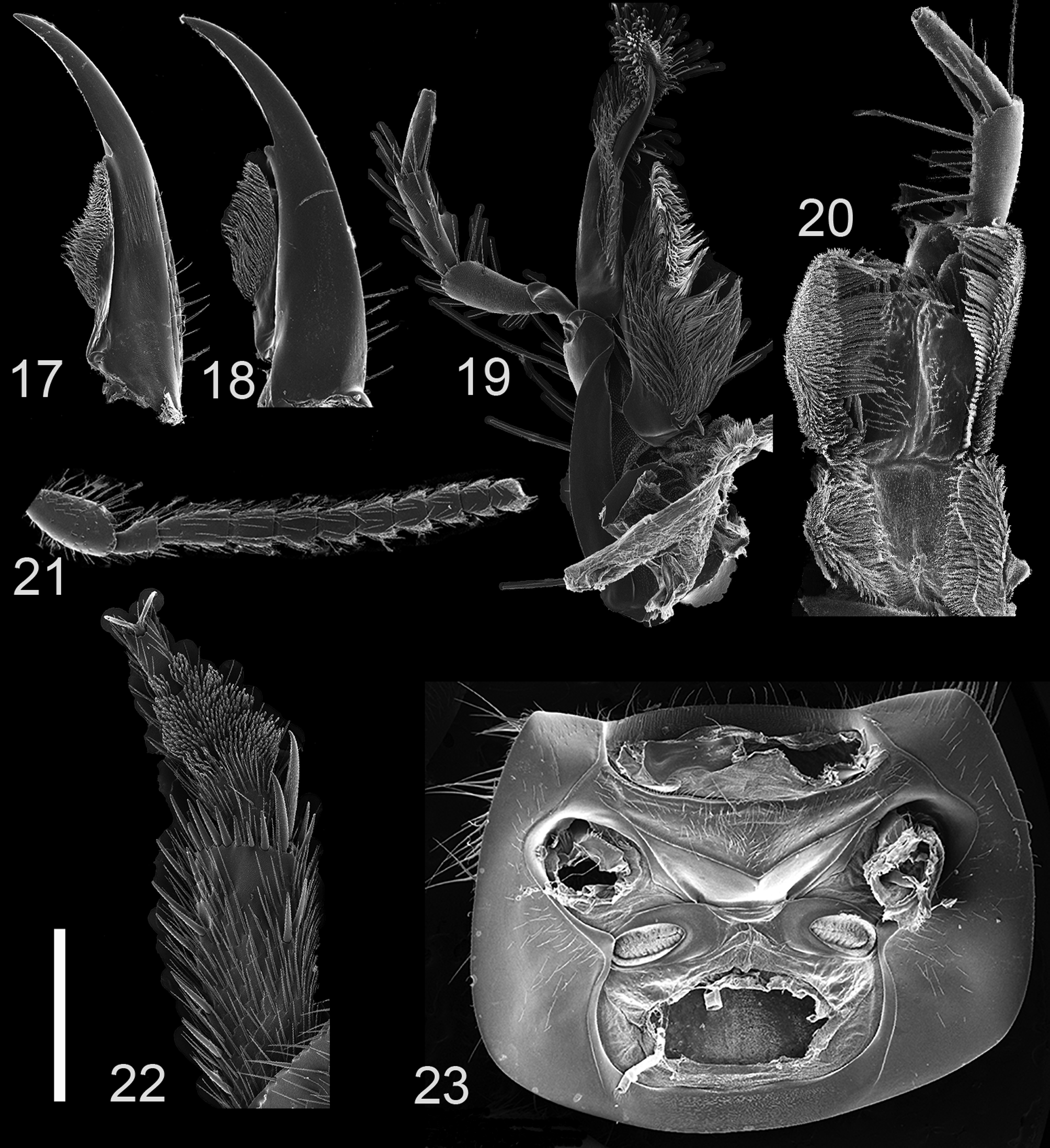
Diagnosis. Due to its peculiar habitus ( Figs. 1–4 View FIGURES 1 – 2 View FIGURES 3 – 4 ), Smilax is a distinct genus that cannot be easily confused with other Xanthopygina genera. Below is a list of morphological features that can help diagnose Smilax : (a) head transverse, rounded; (b) antennae compact, flattened; (c) mandibles long; (d) galea long, as long as maxillary palps; (e) nuchal ridge present; (f) body appearing “fuzzy” due to the presence of long setae; (g) pro-, meso- and metatibia flattened and with two long apical spurs, and (h) abdominal terga III–IV with curved (arch-like) ridge.
The only other Xanthopygina genus that might be confused with Smilax is Haematodes Laporte. Haematodes can be distinguished from Smilax due to its bright red coloration on the head and pronotum, in addition to differences in characters (a), (c), (e) and (h) listed above. Besides Haematodes , there are a few myrmecophile genera in Philonthina (still undescribed) that have a similar pronotum and elytra to Smilax , but their head shape is rather different (smaller) in addition to the typical differences between Philonthina and Xanthopygina (e.g., Smetana and Davies 2000). These taxa are rather similar to Quedius (Pridonius) iheringi Bernhauer but all of these taxa seem to be associated with Eciton Latreille (army ants) instead of Atta Fabricius (leaf cutter ants).
Description. Habitus as in Figs. 1–4 View FIGURES 1 – 2 View FIGURES 3 – 4 ; medium-sized body, robust, with telescopic abdomen, 7.0– 12.5 mm in total length. Coloration of head reddish-brown or metallic dark blue-brown; pronotum metallic green-golden or metallic dark blue-brown, in some species with lateral sides reddish-brown; elytra metallic dark blue-brown to brown; abdomen dark brown to orange. Long macrosetae throughout the body colored lighter than body. Some specimens (including type of S. tuberculata ) appearing dull brown, probably due to loss of metallic coloration (see e.g., Seago et al. 2009). Coloration of abdomen especially variable among specimens of same species.
Head ( Figs. 5–8 View FIGURES 5 – 8 ) transverse, rounded in most species (posterior angles sharper in S. deneinephyto ). Eyes small, positioned anteriorly. Ventral surface of head with microsculpture; postoccipital suture and ventral base ridge present; postmandibular ridge prominent, extending well beyond the postoccular seta; infraorbital ridge pronounced posteriorly; gular sutures separated throughout length, with narrowest point near mid-length; nuchal ridge prominent, forming well defined neck. Epicranium with transverse and polygon-shaped microsculpture, with dense punctation especially on lateral and posterior sides; with large macrosetae around lateral margins. Anteclypeus well developed; clypeus slightly concave. Antenna ( Fig. 21 View FIGURES 17 – 23 ) 11-segmented, flat, compact; antennomeres 1–4 with rows of macrosetae; antennomeres 5–11 with macrosetae and microtrichiae but microtrichiae restricted to anterolateral corners; antennomere 1 robust, punctated, about twice as long and twice as wide as antennomere 2; antennomere 2 expanding distally, smaller than antennomere 3; antennomere 3 1.5 times longer than antennomere 4; antennomeres 4–11 triangular (expanding distally), slightly decreasing in size with every antennomere. Mouthparts with labrum emarginate medially. Mandibles ( Figs. 17–18 View FIGURES 17 – 23 ) elongate, symmetrical, slightly curved distally; with deep fold on lateral edge of dorsal side; mandibular prostheca long; each mandible with single small molar. Maxilla as in Fig. 19 View FIGURES 17 – 23 ; galea long, as long as maxillary palp; galea and lacinia densely setose; maxillary palpi 4-segmented, with transverse microsculpture; P1 small, with small triangular process covering margin of P2; P2–P4 elongate; P2 robust, smaller but wider than P3; P2–P3 with several rows of macrosetae; P4 shorter than P3, apically truncate. Hypopharynx as in Fig. 20 View FIGURES 17 – 23 ; labial palpi 3-segmented; P1–P3 with several rows of macrosetae; P1 small, club-like; P2 twice as long as P1, flattened, expanded distally; P3 shorter than P2, apically truncated.
Pronotum ( Figs. 9–12 View FIGURES 9 – 12 ) wider than head; lateral margins concave; pronotum widest at middle. Pronotum without postcoxal process. Pronotal hypomeron expanded but visible only ventrally, except on anterolateral corners; inferior line of pronotal hypomeron present only anteriorly and not throughout length. Pronotum with microsculpture and micropunctures; pronotal margin with large setiferous punctures bearing large macrosetae; anterolateral angles of pronotum prominent. Basisternum with microsculpture and multiple setae; basisternum with anterior marginal depression present; sternocostal ridge present; furcasternum with small pointed medial carina; furcasternum with transverse microsculpture but with no punctures. Elytra transverse, slightly shorter and wider than pronotum; with dense small uniform punctation and several rows of slightly raised larger setiferous punctures. Mesoscutellum triangular, large, with punctation pattern similar to elytra. Hind wings fully developed. Mesoventrite setose; with anterior margin forming “lip”; without median carina and mesoventral process; middle coxae cavity expanded. Metaventrite with small, slightly emarginate metaventral process. Legs with 5-5-5 tarsal segmentation; femora, tibia and tarsi flattened, with dense uniform punctation, covered with setae; pro- and mesocoxae enlarged, bulbous; pro- and mesofemura with row of small spurs on ventral posterior margin near tibia; protibia with ctenidium in distal margin; tibia robust, with multiple rows of medium size spurs along lateral margins; tibia with with two long apical spurs; protarsi enlarged in both sexes; meso- and metatarsomeres lobed, elongate; empodium with two setae.
Abdomen ( Figs. 13–16 View FIGURES 13 – 16 ) with paired protergal glands present; abdominal terga III–V curved (arc-like) ridge and without accessory basal lines. Abdominal sternite VII in males without porose structure but with modified (various degrees of emargination) posterior margin; abdominal sternite VIII in males with U or V-shaped emargination. Abdominal sternite IX with deep V-shaped emargination posteriorly. Abdomen with small uniform dense punctures; each segment (ventral and dorsal) with row of larger setiferous punctures each giving rise to large dark macrosetae (usually 4–6 per segment).
Male and female genitalia typical of Xanthopygina ; spermatheca not sclerotized; aedeagus smaller in comparison to other genera in Xanthopygina ; paramere in lateral view with enlarged ventral projection; paramere much shorter than median lobe.
Distribution. Distributed from Nicaragua to Argentina, southern Brazil and Paraguay. Habitat. Associated with leaf cutter ants in the genus Atta , typically found in the refuse piles or fungus gardens. Some specimens have been collected with flight intercept traps, perhaps indicative of their ability to move between nests.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Staphylininae |
Smilax Laporte, 1835
| Chatzimanolis, Stylianos 2016 |
Cordylaspis
| Blackwelder 1952: 107 |
| Nordmann 1837: 17 |