Lysmata, Risso, 1816
|
publication ID |
https://doi.org/10.1111/zoj.12044 |
|
persistent identifier |
https://treatment.plazi.org/id/03C787FD-FFA2-957A-FC1E-40047F0799C8 |
|
treatment provided by |
Marcus |
|
scientific name |
Lysmata |
| status |
|
GENUS LYSMATA
While attempting to obtain COI and 12S sequences during this study, problems were experienced in ~25% of the species studied. In most of the above (problematic) species, a single clear band of the expected size was observed during electrophoresis of PCR products. Nonetheless, sequencing of the PCR products and subsequent chromatogram analysis demonstrated the existence of double peaks both in forward and reverse COI and 12S sequences. In some of the same species the chromatograms were reliable (clear and readable) immediately after the primers, but the signal then deteriorated, usually at the same location in both forward and reverse sequences. Importantly, the isolation of PCR bands of the proper size from gels also failed to generate clean sequences, even after repeated attempts (both in COI and 12S). Altogether, this suggests that in shrimps from the genus Lysmata , paralogous sequences are co-amplified with the targeted mtDNA ( COI and 12S) with the sets of primers used here (see Williams & Knowlton, 2001) .
Importantly, cloning of COI PCR products from one of the problematic species ( L. seticaudata ) demonstrated the presence of various indels and stop codons. Also, the analysis of amino acid usage of these cloned sequences indicated considerable variability in amino acid composition. Compositional bias among the cloned sequences was also much more accentuated than that found in the actual COI sequences from other species of Lysmata , Exhippolysmata, and Merguia , and from other crustaceans in other families (e.g. Macrobrachium and Exopalaemon ) and orders (crayfish). The composition bias of the later sequences was minimal. The existence of: (1) multiple bands on › gels of PCR products (as observed in this study), (2) double peaks, background noise, and ambiguity in sequence chromatograms of PCR products that produced a single clear band, and (3) indels, stop codons, and considerable composition bias in cloned sequences of the problematic species L. seticaudata , is herein interpreted as evidence of non-functional numts of the targeted COI mitochondrial gene fragment in shrimps from the genus Lysmata (see Song et al., 2008; Buhay, 2009).
Admittedly, evolutionary processes other than nuclear integration of mtDNA might also explain these results. For instance, heteroplasmy (i.e. the presence of more than one type of mitochondrial genome within a single individual; Frey & Frey, 2004) has been suggested to explain the existence of multiple COI copies in insects ( Frey & Frey, 2004). Usually, heteroplasmy is suspected when cloned sequences are very similar to the targeted mtDNA, and if stop codons and indels are not present in the cloned sequences ( Frey & Frey, 2004; Song et al., 2008). In L. seticaudata , the cloned sequences were remarkably dissimilar from non-numt COI sequences, and the former sequences contained a relatively large number of stop codons. Thus, heteroplasmy does not appear to explain the existence of multiple copies of COI in L. seticaudata .
A second process that might explain the existence of multiple COI -like sequences in L. seticaudata is duplication within the mitochondrial genome (Campbell & Baker, 1999). However, mtDNA gene duplication has not been reported for caridean shrimps in which the mitochondrial genome has been sequenced, and gene duplication is not common in the mitochondrial genome of crustaceans (but see Segawa & Aotsuka, 2005). It is also considered unlikely, but cannot be ruled out, that Taq polymerase errors during PCR reactions are responsible for the existence of multiple COI -like sequences in L. seticaudata (see Williams & Knowlton, 2001). However, the precision of the Taq polymerase used here is high (mutation frequency bp –1 /duplication = 8 ¥ 10 –6), and previous studies suggest that sequences that differ by up to 5 bp (and much less, but not more) are probably the result of Taq polymerase errors (Williams & Knowlton, 2001; Williams et al., 2001). The differences between the two pairs of sequences reported herein exceed this limit. Although alternative processes causing sequencing of multiple copies of mtDNA need to be explored in more detail in the species studied, the available information suggests the existence of COI numts, and probably 12S numts, in the shrimp species studied.
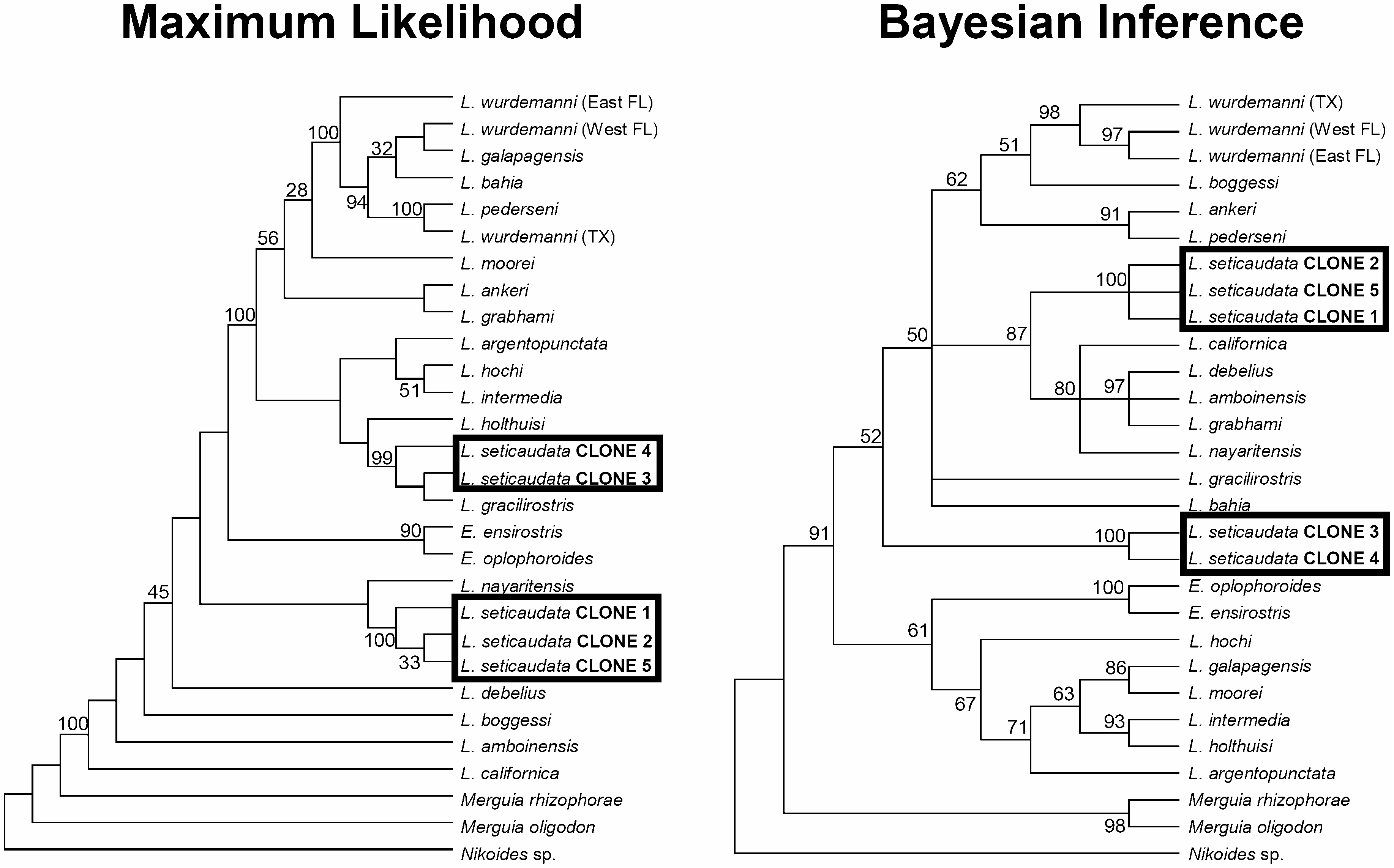
A preliminary phylogenetic analysis using both numts and actual COI ortholog sequences from various species of Lysmata segregated numts into two clades within the phylogenetic tree retrieved. One of the clades was highly divergent from the other numt sequences that clustered together, as well as from the remaining ortholog COI sequences obtained from other species. This strong dissimilarity among numt sequences and their position in the phylogenetic tree suggests that more than a single transfer event from the mitochondrial genome to the nucleus have taken place in L. seticaudata (as reported previously in mammals, sponges, and crustaceans, among others; Fukuda et al., 1985; Bensasson, Zhang & Hewitt, 2000; Williams & Knowlton, 2001; Song et al., 2008; Erpenbeck et al., 2011). Alternatively, a unique transfer occurring in the distant past followed by numt duplication after translocation to the nuclear genome might explain the observed highly divergent numt sequences in L. seticaudata ( Tourmen et al., 2002; Bensasson, Feldman & Petrov, 2003; Kim et al., 2006). Unfortunately, the information generated herein does not allow us to distinguish between these two non-mutually exclusive hypotheses. Additional cloning of numt-like COI sequences in L. seticaudata and in other species might help to reveal the number of transfer events from the mitochondrial genome to the nucleus and numt duplication after translocation in shrimps from the genus Lysmata .
Importantly, considering the remarkable morphological similarity between L. seticaudata and L. intermedia ( D’udekem D’acoz, 2000), it could be expected that the least derived numts of L. seticaudata would cluster together and/or form part of a monophyletic clade containing L. intermedia . Nonetheless, in contrast to our expectations, none of the COI numts from L. seticaudata clustered together with L. intermedia and formed a single well-supported clade. This lack of segregation illustrates the extent of systematic uncertainty that is caused if a combination of numts and mtDNA sequences are used during phylogenetic analysis. If numts were unknowingly amplified in L. seticaudata instead of the targeted COI gene fragment, any phylogenetic analysis would be flawed: the species would cluster together with unrelated taxa, incorrectly indicating morphological convergence among species from different monophylectic clades. Furthermore, two of the highly derived numts (that clustered together) were segregated from other COI sequences by way of a long branch (see trees in Fig. 2 View Figure 2 ), suggesting that the use of a combination of highly derived numts and actual COI sequences might additionally create long branch attraction problems during phylogenetic analyses ( Kennedy et al., 2005). Numts can be, and have been, unknowingly amplified and assumed to be the targeted orthologous mtDNA sequence ( Song et al., 2008, Buhay, 2009). Theoretically, it is even possible to sequence highly derived numts if mutations have not accumulated (by chance) in the region targeted by primers, but have repeatedly taken place and intensively accumulated in the region located among primers of the nuclear translocated sequence. If highly derived numts (unknowingly amplified) together with actual COI sequences have been used for phylogenetic reconstruction in these shrimps, the phylogenetic analysis(es) would have suggested inaccurate (but perhaps robust) relationships among the species studied.
Finally, COI (and probably 12S) numts appear to be pervasive in the genus Lysmata , as problems were experienced in ~25% of the targeted species when attempting to obtain COI and 12S sequences. This high prevalence of COI (and probably 12S) numts in the genus Lysmata creates a serious challenge for the development of a DNA barcode in these valuable ornamental shrimps ( Calado 2008). It has become imperative to develop a DNA barcode for Lysmata and related genera given their traded volume, unknown exploitation levels of various species from coral reef environments, and the pervasive mislabelling of these shrimps in the aquarium trade (J.A. Baeza, unpubl. data). Numts of recent origin within a species often appear sister to functional sequences, whereas numts that arose earlier in the phylogeny of a clade often appear as basal clusters found across many species ( Arctander, 1995; Buhay, 2009). Thus, a first problem arising from the existence of numts is that DNA barcoding analyses incorrectly overestimate the number of evolutionarily significant units and unique species based on the standard metric of 3% sequence divergence (see Song et al., 2008). Furthermore, DNA barcoding strives for rapid and inexpensive generation of molecular species tags ( Song et al., 2008; Buhay, 2009). This will certainly be not the case if a DNA barcode is developed in Lysmata using COI or 12S genes. COI has been successfully used as a barcode in a wide range of marine and terrestrial vertebrates and invertebrates ( Hebert et al., 2003; Waugh, 2007); however, in other groups, this gene fragment does not work well, and other mitochondrial or nuclear markers have been proposed as alternatives ( Waugh, 2007; Buhay, 2009 and references therein). Herein, we suggest that the mtDNA 16S gene might be used as a barcode alternative in ornamental shrimps given the apparent absence of numts in this marker and high information content (see below). Indeed, previous studies using 16S as a barcode have successfully differentiated among cryptic species complexes in the genus Lysmata , resulting in the description of new species and validation of other species differentiated from one another by minimal but consistent morphological traits (e.g. L. udoi, Baeza et al., 2009b ; L. grabhami versus L. amboinensis, Baeza, 2010a ). Studies exploring the feasibility of the mtDNA 16S gene as a barcode in various ornamental crustacean genera (e.g. crabs from the Mithrax –Mithraculus species complex, and shrimps from the genera Lysmata , Periclimenes , and Saron , among others) are currently underway.
| COI |
University of Coimbra Botany Department |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.