Anthicinae, Latreille, 1819
|
publication ID |
https://doi.org/10.37520/aemnp.2020.007 |
|
publication LSID |
lsid:zoobank.org:pub:7990B912-A3D4-40F7-B143-772FFDB5A119 |
|
persistent identifier |
https://treatment.plazi.org/id/03C8343F-AA2C-1071-FF65-8B97C62AFA76 |
|
treatment provided by |
Tatiana |
|
scientific name |
Anthicinae |
| status |
|
I. Anthicinae (tribal classification), mesothoracic form and function, canthariphily
The subfamily Anthicinae Latreille, 1819 is currently composed of three tribes: Anthicini , Formicomini Bonadona, 1974 , and Microhoriini . A fourth tribe, the monotypic Endomiini Kaszab, 1956 based on Endomia Laporte, 1840, was placed as a junior synonym of Anthicini by BONADONA (1974) without comment, which was followed in a few later papers ( BONADONA 1976, 1991, 2013; BUCCIARELLI 1977), or not followed for reasons unexplained ( BUCCIARELLI 1980). This synonymy is accepted here, as Endomia is undoubtedly close to Anthicus Paykull, 1798 ( BONADONA 1976), sharing both aedeagal and important external characters (mainly structure of the mesothorax). The genus is distinct by the presence of the lateral lobes of the frons covering the antennal bases (bases not covered in other Anthicini ), the lack of an antebasal sulcus of pronotum (variably distinct within the Anthicini ), and reduced terminal spurs of tibia (though present); the short, scale-like setae of the body have been shown to transition to longer, aciculate setae as seen in Endomia kejvali Degiovanni, 2016. We treat this combination of features as strong support for recognizing the genus Endomia, but not for treating this genus as the sole member of a separate tribe.
Major characters for grouping into tribes reside with the genitalic characters ( BONADONA 1958a, 1974, 1990 a, 2013; BUCCIARELLI 1980; CHANDLER 2010), but some external characters can be used as sets for accurately placing genera into tribes without resorting to examination of the male genitalia.
Anthicini : (i) mesepimera almost exclusively distinctly impressed to excavate and frequently distinctly setose; (ii) tibial spurs (especially mesotibial) mostly spinulose; (iii) intercoxal process of first abdominal ventrite more or less distinctly pointed to rounded apically (very rarely subtruncate) in dorsal view, its marginal bead almost exclusively complete; (iv) elytra with sutural stria present on at least apical third; (v) tegmen always open ventrally and clearly divided into parameral plate and phallobase, phallobase flattened, its basal margin truncate ( Fig. 3 View Figs 1–6. 1 ); (vi) penis distinct, baculi of penis more or less widely separated (fused in some Endomia), primary gonopore slight; (vii) male elytral apices simple, channel/pores for cantharidin gland absent, species rarely canthariphilous, if canthariphilous then both sexes equally [?] attracted.
Formicomini : (i) mesepimera with small, nude fovea posteriorly, at most slightly impressed (never excavate); (ii) tibial spurs smooth; (iii) intercoxal process of first abdominal ventrite always broadly rounded to subtruncate, its marginal bead interrupted apically; elytra with sutural stria present on at most apical third; (v) tegmen always entirely open ventrally and clearly divided into parameral plate and phallobase, phallobase somewhat convex, its basal margin rounded ( Fig. 4 View Figs 1–6. 1 ); (vi) penis distinct, baculi fused into elongate apodeme (at least in basal half), base of apodeme simple, primary gonopore distinct, with small opening; elytral apices simple, channel/pores for cantharidin gland absent, species rarely canthariphilous, if so both sexes attracted.
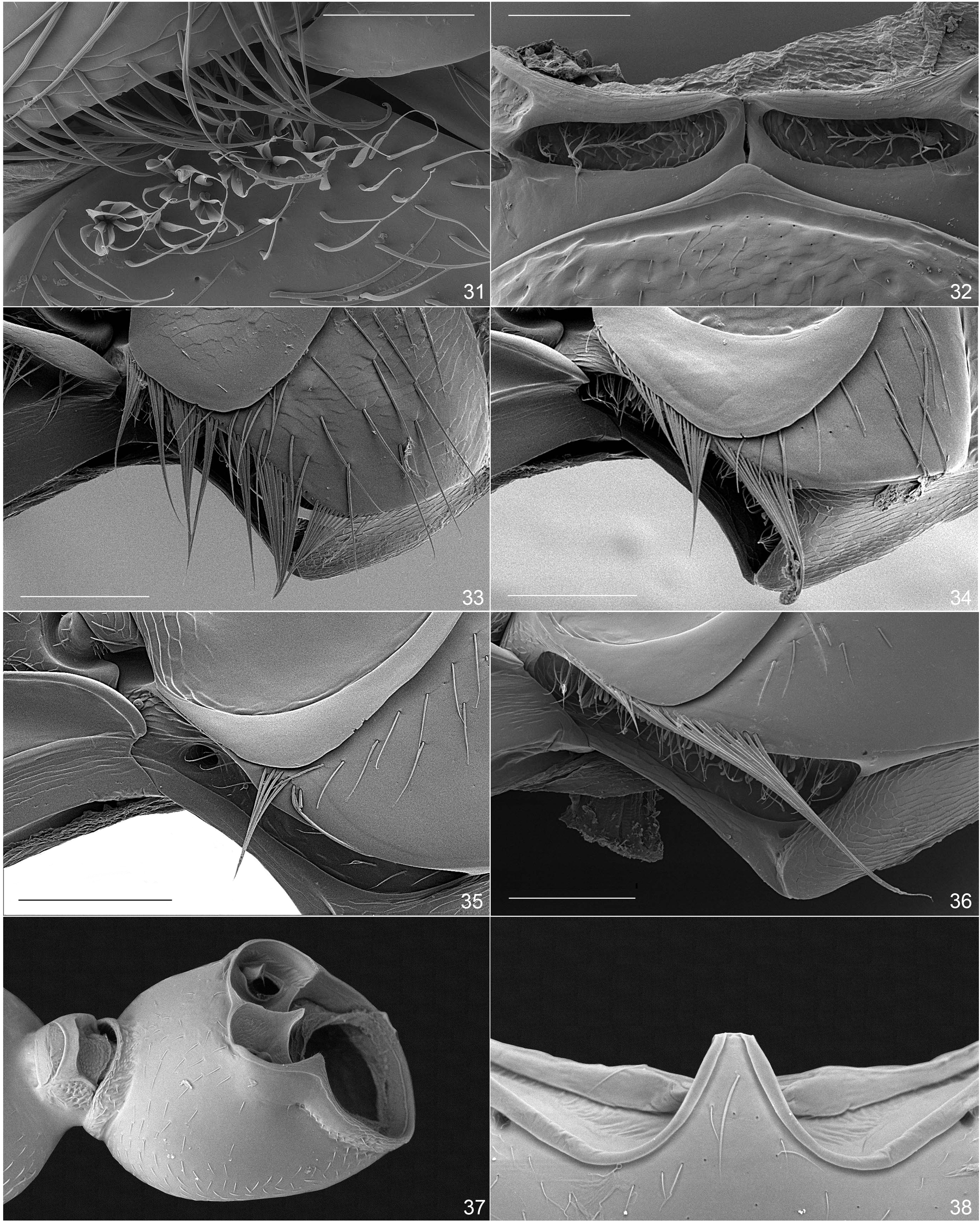
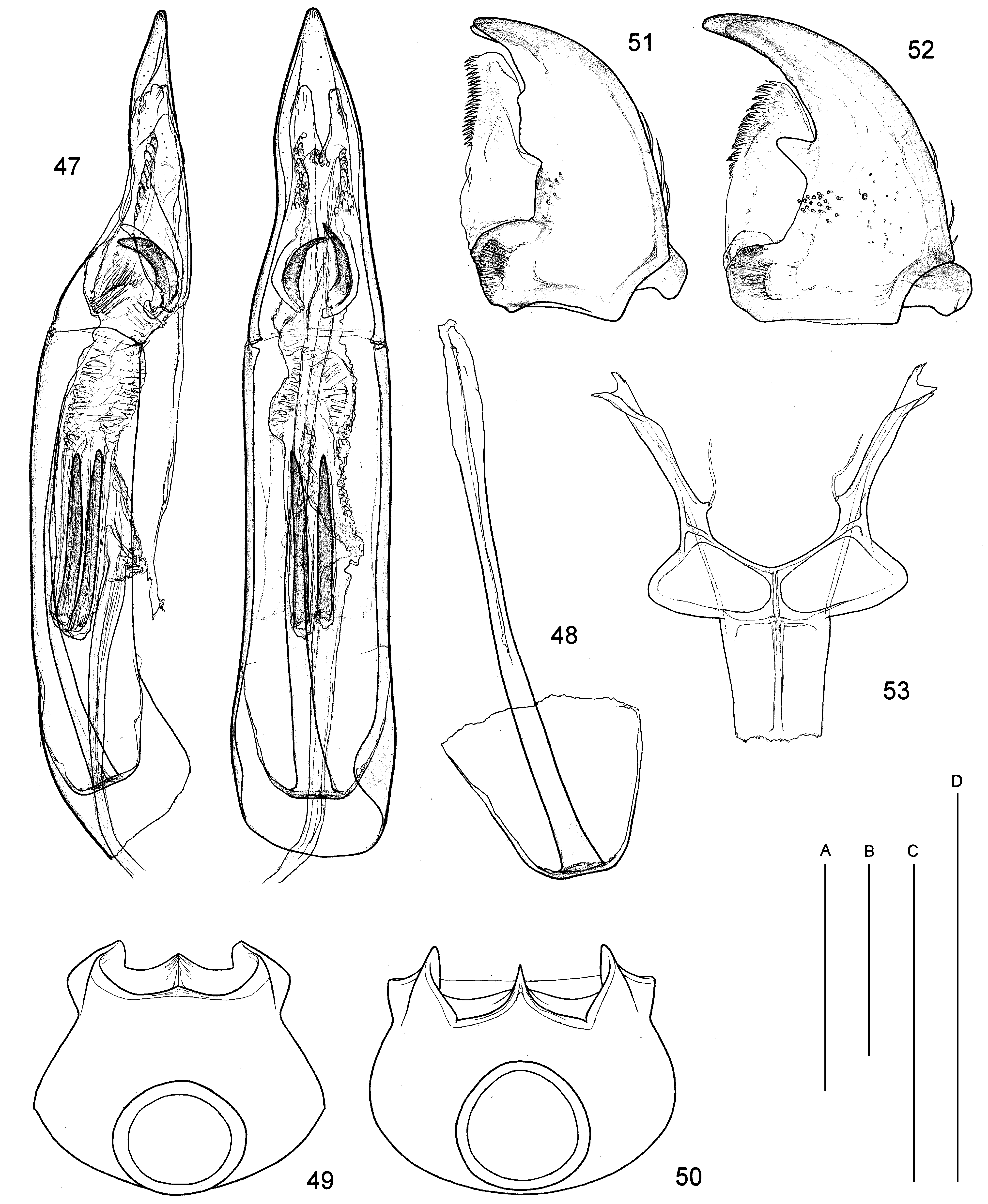
Microhoriini : (i) mesepimera excavate ( Figs 35, 36 View Figs 31–38. 31 ), except Falsophilus and some apterous Aulacoderus ; (ii) tibial spurs smooth; (iii) intercoxal process of first abdominal ventrite variable in form but its marginal bead almost exclusively complete, except Falsophilus ( Fig. 38 View Figs 31–38. 31 ); (iv) elytra with sutural stria lacking to slightly indicated near apex; (v) tegmen partly closed, circular (sleeve-like), encasing endophallus ( Figs 5, 6 View Figs 1–6. 1 ), exceptionally open ventrally but then strongly convex ( Neocrohoria, Fig. 47 View Figs 47–53. 47, 48 ), separation of parameral plate and phallobase either weakly indicated or indistinct (mostly); (vi) penis indistinct, baculi forming short to long thin free apodeme, base of apodeme attached to membranous apically-facing ‘cup/plate’ ( Fig. 48 View Figs 47–53. 47, 48 ), primary gonopore usually well-developed with opening large, but may be also indistinct; (vii) elytra almost exclusively with apical modifications related to presence of channel/ pores for cantharidin gland (varying in prominence, Figs 41–46 View Figs 39–46. 39, 40 ), many species are canthariphilous, with only the males being attracted.
Mesothoracic form and function. BONADONA (1958a: 7–8) was the first author to fully present characters of the ventral sclerites of the mesothorax when he systematically illustrated and discussed its structure for the genera treated in his monograph of the Madagascan Anthicidae . He continued providing these data for genera in some following papers: the African/Madagascan Tomoderini ( BONADONA 1961), the genera of Notoxinae of France ( BONADONA 1971), and for several other genera that occur in France ( BONADONA 1974). Mesoventral characters were later used also by WERNER & CHANDLER (1995) to characterize groups of the New Zealand Anthicinae . Morphology of the mesoventrite, mesepisterna, and mesepimera have been shown to be valuable in characterization of subfamilies, tribes, generic clusters, and genera of Anthicidae , with our focus being on the characteristics of the Microhoriini .
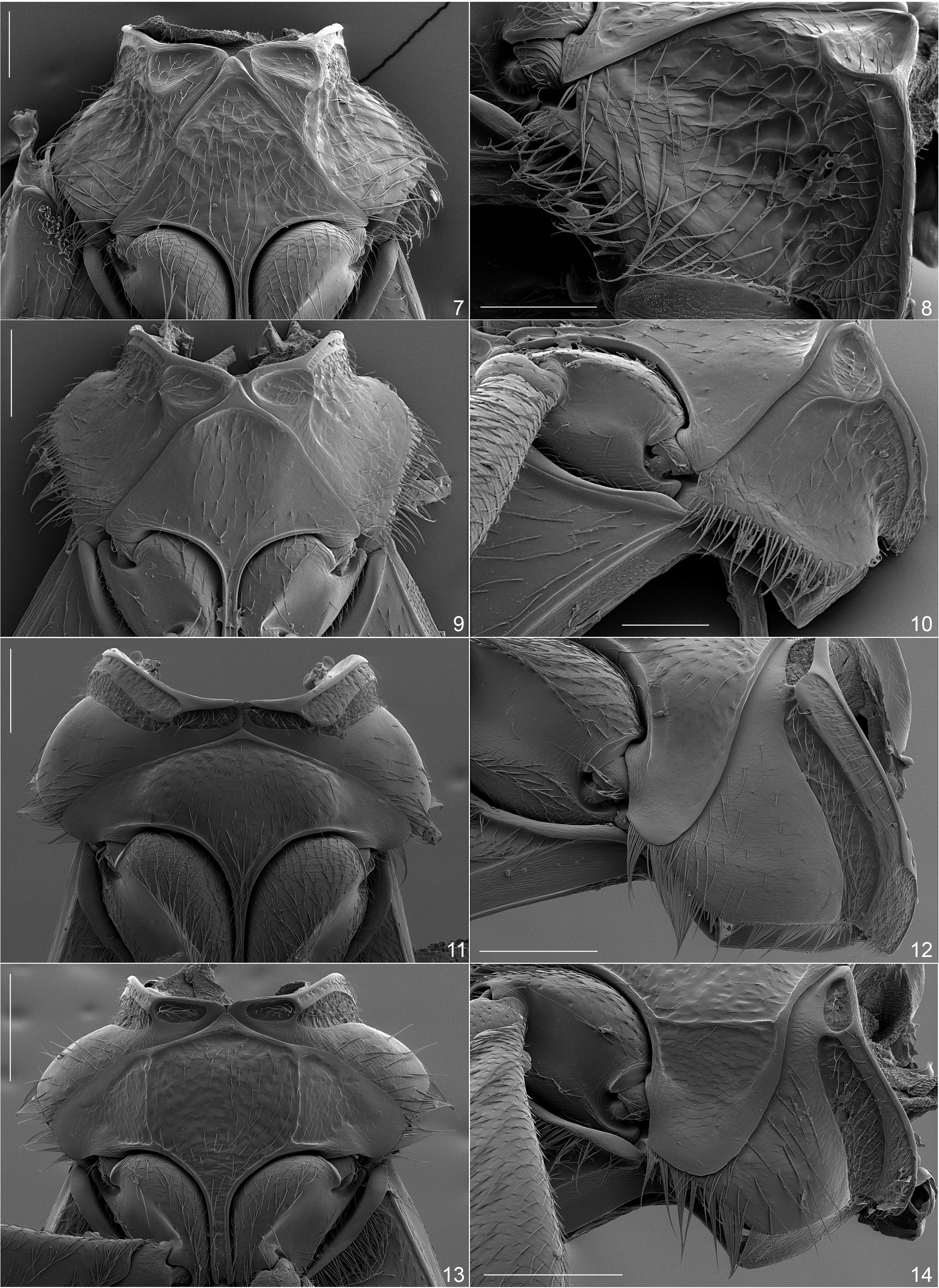
Mesoventrite. The basic form of the mesoventrite is triangular with the lateral margins straight and convergent anteriorly to an acute median point ( BONADONA 1974; BUCCIARELLI 1980; WERNER & CHANDLER 1995; CHANDLER 2002, 2009; LAWRENCE & ŚLIPIŃSKI 2013). The posterolateral angles are narrowly rounded and acute, and extend no further laterally than to the outer margins of the mesocoxal cavities; medially it extends posteriorly as the mesoventral process that separates the mesocoxal cavities. This shape is typical of Pyrochroidae , Ischaliidae , Meloidae ( Eleticinae and members of other subfamilies), and basal subfamilies of Anthicidae ( Eurygeniinae , Steropinae ), with Copobaeninae and Macratriinae being differently modified from this basic form. In Anthicinae there are genera/generic clusters that have the lateral margins of the mesoventrite strongly, laterally lobed, with the posterolateral angles broadly rounded and extending laterally beyond a point even with the lateral margins of the mesocoxal cavities. One of the generic clusters with the lobed mesoventrite is found in the Microhoriini . Here the ancestral form with straight lateral margins is present for the genera Aulacoderus , Falsophilus, and Neocrohoria ( Figs 7, 9 View Figs 7–14 ), with the derived lobed form characterizing the genera Liparoderus LaFerté-Sénectère, 1849 and Microhoria ( Figs 11, 13 View Figs 7–14 ).
Mesepisterna. The apical portion of the mesothorax is weakly to strongly constricted near the point of articulation with the pronotum to form a more or less broad apical ‘neck.’ The anterior margin of the mesothorax is formed by the apices of the mesepisterna that are medially touching or fused to different extents. The procoxal rests present in this area may be connected to or isolated from the anterior thickened rim of the mesepisterna, and the area immediately posterior to this rim can be shallowly impressed (ancestral for Anthicinae ; microhoriine genera Aulacoderus , Falsophilus, Neocrohoria; Figs 8, 10 View Figs 7–14 ) or modified to form deep, transverse, well-defined lateral grooves (derived; microhoriine genera Liparoderus and Microhoria ; Figs 12, 14 View Figs 7–14 ). There are analogous but different modifications of the mesepisterna in the other Anthicinae groups, mainly in those with lobed mesoventrites.
Mesepimera. The basal Anthicidae (e.g. Steropinae , Copobaeninae , and Eurygeniinae ) and closely related heteromeran families (e.g. Meloidae , Pyrochroidae ) have large mesepimera that are quite distinct, lack lines of longer setae on the posterior margin, lack any marginal foveae, and if they are tightly connected with mesepisterna, the suture is clearly defined, except for some Stereopalpus LaFerté-Sénectère, 1849 and for Mitraelabrus Solier, 1851 ( Eurygeniinae ).
In Anthicinae the mesepimera are always inconspicuous, being narrow and smoothly fused to the mesepisterna, with the line of fusion often seen internally as a linear flange, meaning that the apparent outer portion of the mesepisterna is in reality the mesal portion of the mesepimera. In addition they always have one or two internally projecting foveae at the sides facing the metepisterna, which is more or less deeply impressed and frequently conspicuously setose on/ along the postero-ventral margin. These mesepimeral setae may be modified in various ways that are useful in recognizing generic clusters. If well-developed, the postero-lateral impression/cavity of mesepimera can be distinctly setose, including some thicker, specialized setae ( Figs 34, 36 View Figs 31–38. 31 ).
The function of mesepimeral foveae is unknown. They are often small, but can be conspicuously large, as in Africomus Kejval & Chandler, 2016, numerous Sapintus Casey, 1895, and Aulacoderus . VAN HILLE (1984) discussed presence and form of these foveae in defining his species-groups of Aulacoderus , with their differences being included in his keys and species descriptions.
Mesothoracic glands. The functions of the laterally lobed mesoventrite and transverse mesepisternal grooves that are characteristic of Microhoria sensu novo and Liparoderus have not been wholly determined. However, the anteromedial margin of the mesoventrite is immediately posterior to the opening of the mesothoracic glands where iridoid compounds are released and which probably repel ants and other potential predators (see below). HEMP & DETTNER (1997) theorized that the channel formed at the juncture of the mesoventrite and mesepisterna/mesepimera could serve as a conduit to disperse the iridoid compounds laterally through capillary action to the point where the channel contacts the mesepimeral setal row. Here the row of dense or spaced setae can serve as dispersing structures for the iridoids by wicking the compounds up the setae so they may volatilize quickly, with the strong curve of the mesoventrite allowing placement of a larger number of setae on the mesepimera to disperse the chemicals. Certainly many species of Acanthinus, seem to run or forage with ants without being attacked or even noticeably disturbed (D. S. Chandler, pers. observ.), which could be attributed to the chemical dispersal potential of the bowed mesoventrites and the mesepimera with their long appressed to erect setae.
The mesothoracic glands have been found in all members examined of the Anthicinae , Notoxinae , and Tomoderinae ( HEMP 1994, DE MARZO 2006, C. Hemp, in litt. [note: De Marzo did not find them in Aulacoderus , though other authors did, see below]). Size and morphology of these glands, chemical composition of their secretions and position of the gland orifice are variable ( HEMP 1994, HEMP & DETTNER 1997, DE MARZO 2006). Different compounds are produced at the level of subfamily, with these glands producing various iridoid compounds and/or their precursors by members of the Anthicinae , aromatic compounds by Notoxinae , and indols by Tomoderinae ( HEMP 1994; C. Hemp, in litt.). The defensive role of the iridoids was determined by laboratory tests and field observations in species of Anthelephila Hope, 1833 ( HEMP & DETTNER 1997). Glands of Microhoriini were found to be moderately large in Microhoria terminata and small in Aulacoderus species ( HEMP 1994, HEMP & DETTNER 1997). In an unpublished study by Claudia Hemp (in litt.) the mesothoracic glands of 18 species of Microhoria (mesoventrite laterally lobed) and four species of Aulacoderus (mesoventrite laterally straight) were examined, with all Microhoria measured and having ‘huge’ glands, and all Aulacoderus having ‘small to medium’ glands, with the correlation of laterally lobed mesoventrites with large glands being quite strong.
It is interesting that of the families that produce or consume cantharidin only the closely related Meloidae and Anthicidae have been documented as having mesothoracic glands ( HEMP & DETTNER 1997). The defensive compounds produced by the mesothoracic glands act to protect the adults; for those species that do consume cantharidin, adults will be protected by the iridoid secretions whether or not they find cantharidin, but for those with access to a cantharidin source the eggs, larvae, and pupae will be protected also.
Canthariphily. Cantharidin is a monoterpene anhydride naturally produced by the fat body of many members of the Meloidae and Oedemeridae ( HOLZ et al. 1994, DETTNER 1997, HASHIMOTO et al. 2016, JIANG et al. 2017). The compound is quite toxic to insects and vertebrates. Its presence is often coupled with strongly marked color patterns associated with aposematic coloration and serves as a potent feeding deterrent ( CUÉNOT 1890, GÖRNITZ 1937, VAN HILLE 1954, CARRELL & EISNER 1974, DETTNER 1997). Meloid males are the primary producers of cantharidin, which is passed to the females in spermatic fluids during mating ( SIERRA et al. 1976). The cantharidin is then passed by the females into their eggs ( DETTNER 1997, NIKBAKHTZADEH et al. 2007) ensuring that this feeding deterrent is present not only in the female, but also serves to protect the eggs and subsequent larvae from predators and fungi (MCCORMICK & CARRELL 1987, DETTNER 1997).
Though few insect groups produce cantharidin, its defensive properties have been appropriated by many groups by ingesting fluids and/or portions of living, moribund, and dead meloid and oedemerid beetles for a similar if derivative function ( YOUNG 1984a, HEMP & DETTNER 2001). Relevant to this paper is that Anthicidae are the most abundant and diverse group of beetles attracted to cantharidin. Compilations of canthariphilous species have been produced by ABDULLAH (1965a), YOUNG (1984a), and HEMP & DETTNER (2001), and indicate that members of the Microhoriini and Notoxinae are the most diverse and abundant beetles attracted to cantharidin. This is in contrast to the Anthicini and the other tribes/subfamilies of Anthicidae , where scattered positive associations have been noted, but for many of these species their abundances are low. GUYON (1848) was the first to note this phenomenon for Anthicidae when he recorded Notoxus monoceros (Linnaeus, 1760) feeding on Oedemera lurida (Marsham, 1802). TYLDEN (1865) provided the earliest record for meloids when he reported several N. monoceros feeding on a dead Meloe proscarabaeus Linnaeus, 1758. Among the following succession of records during the 1800’s were notes establishing the strong attraction of members of the Microhoriini to meloids: Liparoderus insignis (Lucas, 1843) ( SANZ DE DIEGO 1880, BOLIVAR Y URRUTIA 1896), Microhoria fairmairei ( CHOBAUT 1895), Microhoria aubei, Microhoria chobauti, Microhoria cinctuta, and Microhoria pumila ( PIC 1896; all as Anthicus ), with many subsequent records summarized by YOUNG (1974) and HEMP & DETTNER (2001).
Members of the Microhoriini and many Notoxus Geoffroy, 1762 ( Notoxinae ) have apical modifications of the male elytra where small amounts of cantharidin are secreted to attract females. During mating the cantharidin-rich spermatic fluids carry this compound to the females where some is placed in the eggs, conveying protection to the eggs and larvae from predators as well as fungi ( SCHÜTZ & DETTNER 1992). In the most recent list of canthariphilous species by HEMP & DETTNER (2001), the totals for Microhoriini are: Aulacoderus , 48 species; Liparoderus , two subspecies of L. insignis ; Microhoria sensu novo, 18 species ( oedipus species-group, 10 species; terminata species-group, 5 species; schimperi species-group, 2 species; and ocreata species-group, 1 species). HASHIMOTO & HAYASHI (2014) recently added Microhoria fugiens (as Clavicollis, presently fugax species-group) as a canthariphilous species. The male/ female sex ratio for attracted specimens of these species is usually near or at 100% males ( VAN HILLE 1954, SCHÜTZ & DETTNER 1992, DETTNER 1997, HEMP et al. 1999, HASHIMOTO & HAYASHI 2014). It is interesting that two phylogenetically distant groups of Anthicidae ( Notoxinae and Anthicinae : Microhoriini ) have either independently evolved or primitively retained these elytral glands that use cantharidin as an allomone to reduce predation pressure, with the chemical also acting as a selective agent that increases male reproductive success ( VAN HILLE 1984).
Scattered members of certain genera, such as Anthelephila and some species-groups of Notoxus, may be strongly attracted to cantharidin, but lack the male elytral modifications and have both sexes attracted in roughly equal numbers ( VAN HILLE 1954, ABDULLAH 1965b, DETTNER 1997). For these groups cantharidin appears to function as both an aggregation and sex pheromone ( ABDULLAH 1965b) that brings both sexes together where they actively mate, and also obtain a defensive compound that provides protection to both sexes after they consume their cantharidin source ( DE MARZO 1992, HEMP et al. 1997, DETTNER 1997, HEMP et al. 1999).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.