Catapaguropsis queenslandica, Lemaitre, Rafael & Mclaughlin, Patsy A., 2006
|
publication ID |
https://doi.org/ 10.5281/zenodo.173566 |
|
DOI |
https://doi.org/10.5281/zenodo.6260301 |
|
persistent identifier |
https://treatment.plazi.org/id/03C87213-B873-FFE6-CD27-FAC806BF5282 |
|
treatment provided by |
Plazi |
|
scientific name |
Catapaguropsis queenslandica |
| status |
sp. nov. |
Catapaguropsis queenslandica View in CoL n. sp. ( Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 )
Type material. Off Baldina, NE Queensland. Holotype male (sl = 2.1 mm), R/V Franklin stn 422, 17°21.8’S, 146°48.5’E, 303– 296 m, 15 May 1986 (W16589).
Paratype. Ovig. female (sl = 1.9 mm), same data as holotype.
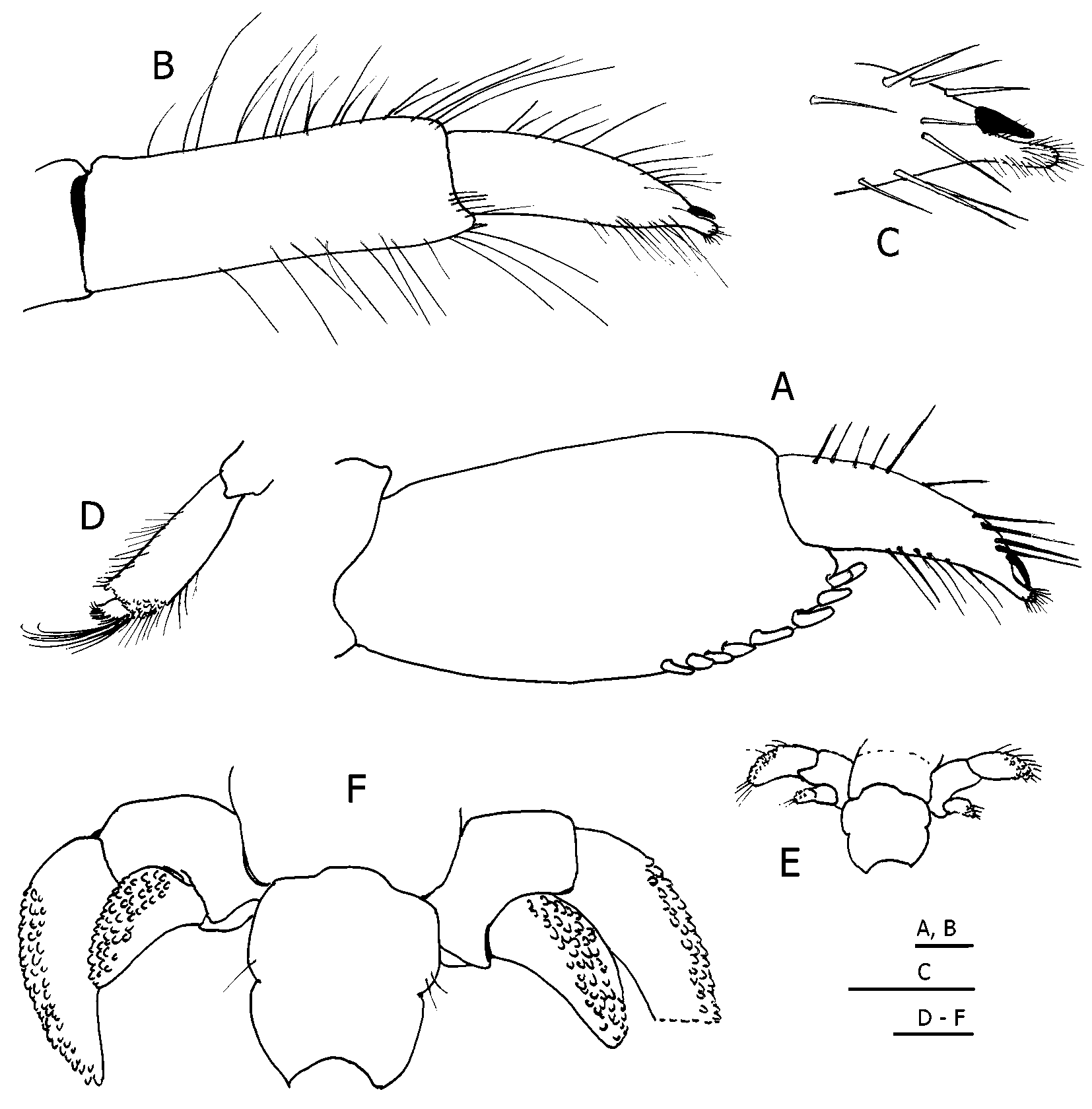
Description. Shield ( Fig. 1 View FIGURE 1 A) somewhat vaulted, broader than long, weakly calcified; anterior margin between rostral lobe and lateral projections concave; anterolateral margins sloping; posterior margin roundly truncate. Rostral lobe broadly rounded or roundly subtriangular, produced little if at all beyond level of lateral projections, latter unarmed or with tiny terminal spine. Carapace lateral lobes elongate, reaching anterior 0.3 of shield. Posterior carapace short, with broad median plate; cardiac sulci reaching to posterior margin. Branchiostegites membranous, unarmed.
Ocular peduncles very short, only slightly more than half length of shield; corneal diameter approximately equal to total peduncular length (including cornea). Ocular acicles triangular, reaching slightly beyond proximal margins of ultimate peduncular segments in female ( Fig. 1 View FIGURE 1 B), shorter in male, each terminally acute or with tiny submarginal spine; separated basally by more than twice basal length of one acicle.
Antennular peduncles overreaching distal margins of corneas by more than lengths of ultimate peduncular segments; ultimate segment with few long, stiff setae on distal margin; penultimate segment with few scattered setae; basal segment with unarmed dorsolateral margin.
Antennal peduncles overreaching distal corneal margins by approximately 0.5 lengths of ultimate segments. Fifth and fourth segments unarmed; third segment with small ventral spine; second segment with dorsolateral distal angle produced, terminating in small spine, dorsomesial angle with small spine; first segment unarmed. Antennal acicle reaching distal margin of fourth peduncular segment, slender, terminating in simple spine. Antennal flagella missing. Third maxilliped with prominent spine on dorsodistal margin of merus.
Chelipeds subequal in length, but right appreciably stouter; both lacking hiatus between dactyl and fixed finger. Right cheliped ( Fig. 3 View FIGURE 3 A–C, F) with chela 2.6 (female) to 3.2 (male) as long as broad; left chela 5.5 (female) to 7.3 (male) as long as broad. Dactyl 0.5 (male) to 0.6 (female) length of palm; dorsomesial margin rounded, dorsal surface weakly convex, all surfaces unarmed, but with numerous scattered, moderately long setae ventrally; cutting edge serrate ( Fig. 3 View FIGURE 3 C) with 2 low, broad, calcareous teeth, terminating in tiny corneous claw, slightly overlapped by fixed finger. Palm slightly longer than carpus, dorsomesial and dorsolateral margins rounded and unarmed, dorsal surface weakly convex, also unarmed, fixed finger similarly unarmed, but ventral surfaces of both palm and fixed finger with numerous moderately long setae; cutting edge of fixed finger serrate ( Fig. 3 View FIGURE 3 C) with 2 broad, low calcareous teeth, terminating in tiny corneous claw. Carpus slightly longer than merus; dorsomesial and dorsolateral margins each with row of irregularlysized spines, largest in male; male with 1 very small spine on distomesial margin, ventromesial and ventrolateral margins each with row of tiny spines; female without spines on distomesial margin or either ventral margin. Merus laterally compressed; male with prominent spine on dorsodistal margin, row of small spines on dorsomesial margin, decreasing in size proximally, mesial face with few spinules ventrally, ventromesial and ventrolateral margins each with row of small spines; female with small spine on dorsodistal margin, other margins and surfaces unarmed. Ischium unarmed in both sexes.
Left cheliped ( Fig. 3 View FIGURE 3 D, G, E) long and very slender. Dactyl approximately 1.5 length of palm longer than palm; surfaces rounded and unarmed, but with very sparsely scattered, moderately short setae; cutting edge with row of tiny, slender corneous teeth, terminating in minute corneous claw and sparse tuft of very short setae. Fixed finger similarly rounded and unarmed but with sparsely scattered setae; cutting edge with 4 minute calcareous teeth interspersed with minute corneous teeth, terminating in minute corneous claw and very sparse tuft of short setae. Palm with convex dorsal surface unarmed and glabrous; dorsomesial and dorsolateral margins rounded. Carpus and merus both noticeably longer than palm but only slightly longer than dactyl; dorsomesial and dorsolateral carpal margins each with row of very small, rather widelyspaced spines, female with dorsodistal spine considerably more prominent; surfaces all unarmed and with very few scattered setae. Merus with very small (male) or prominent (female) dorsodistal spine; surfaces unarmed; ventromesial and ventrolateral margins not delimited. Ischium unarmed, but with 1 or 2 spiniform protuberance on dorsal surface proximally.
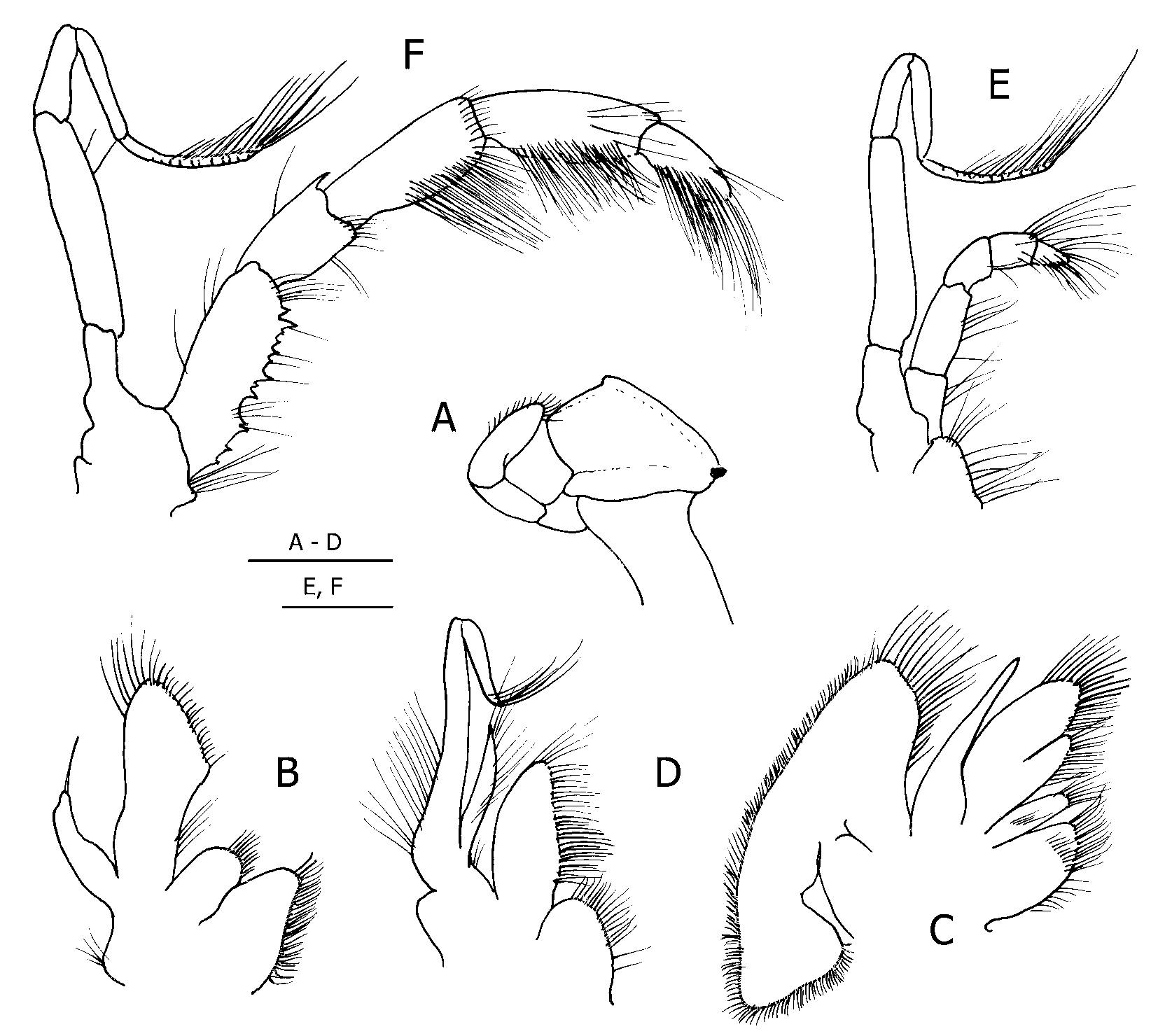
Second and third pereopods ( Fig. 4 View FIGURE 4 ) sexually dimorphic. Male with second pereopods distinctly shorter than third; dactyls long, slender, left second approximately equal to length of propodus, left third 0.9 of propodal length, right second 0.8, right third 0.7 length of propodus, each with both dorsal and ventral row of moderately short, fine setae, all terminating in short, slender corneous claws. Propodi approximately twice length of carpi; surfaces all unarmed but each with dorsal and ventral row of short, fine, closelyspaced setae. Dorsal margins of carpi each with row of short, fine setae, dorsodistal margins each with minute or tiny spinule. Meri nearly three times length of carpi, unarmed, but each with few minute protuberances dorsally. Ischia unarmed. Second and third pereopods of female (second left missing) of approximately equal length. Dactyls 0.7–0.8 length of propodi, somewhat bladeshaped; terminating in small corneous claws; unarmed, but each with dorsal and ventral row of moderately long, fine setae. Propodi approximately twice length of carpi, unarmed but each with dorsal and ventral row of moderately long, fine setae. Carpi approximately 0.8 length of meri; dorsodistal margins each usually with tiny hooked spine, dorsal margins serrate, each with row of moderately short, fine setae. Meri each with small dorsodistal spine and 2 subdistal spines or spiniform protuberances on dorsal margin, also row of sparse setae dorsally. Fourth pereopods of female ( Fig. 5 View FIGURE 5 A) weakly semichelate, propodal rasps each with single row of corneous scales; fourth pereopods of male ( Fig. 5 View FIGURE 5 B) simple, no propodal rasp; preungual process ( Fig. 5 View FIGURE 5 C) present in both sexes, slightly exceeding distal claw on dactyl, with distal portion setose. Fifth pereopods ( Fig. 5 View FIGURE 5 D) similar in both sexes, minutely chelate. Sternites of second and third pereopods each with median concavity, more pronounced in male; anterior lobe of third subrectangular in both sexes.
Male with coxae of fifth pereopods approximately equal, right with stout, short sexual tube appearing as posterior coxal extension ( Fig. 1 View FIGURE 1 D) directed to exterior; coxa of left with very short tube directed posteriorly; pleon markedly reduced posteriorly; no paired or unpaired pleopods. Female with paired gonopores; without paired first pleopods, pleon damaged but with only unpaired pleopods 3 and 4 present, no unpaired fifth; noneyed eggs 0.5–0.6 mm diameter.
Uropods and telson ( Fig. 5 View FIGURE 5 E, F) symmetrical in both sexes, markedly reduced in size in male. Telson with slight lateral incisions separating anterior and posterior portions; posterior lobes separated by broad, Ushaped median concavity; terminal margin unarmed or with 1or 2 microscopic spinules.
Etymology. The specific epithet, queenslandica , is derived from the Australian state of Queensland.
Color. Unknown.
Habitat. Unknown.
Distribution. Known only from the type locality.
Var ia t io n. Although morphological variation cannot be assessed from the present two specimens, marked sexual dimorphism is apparent. Most notable are the difference in lengths of the male second and third pereopods, and the differences in the shapes of the pereopodal dactyls and propodi of the fourth pereopods between the male and female. Differences in the sizes of the uropods and telsons of the male and female also are substantial, but it is not known whether these are adaptations to different habitats or simply reflect abnormal development of these structures in the male holotype.
Remarks. Although sexually dimorphic preferences for particular species or sizes of gastropod shells have been demonstrated for some hermit crab species ( Blackstone & Joslyn 1984, Asakura 1995, Elwood & Kennedy 1988), only two species, Calcinus verrillii ( Rathbun, 1901) , and Calcinus tubularis ( Linnaeus, 1767) , are known to be dimorphic in their basic habitat preferences. In both species, females most commonly occupy attached vermetid and turritellid tubes whereas males are found in typical mobile gastropod shells ( Rodrigues et al. 2000, Gherardi 2004). Neither the study by Rodrigues et al. (2000) nor Gherardi (2004) addressed morphological dimorphism if any was present; however, Rodrigues et al. (2002), in a continuation of their work with C. verrillii , examined the effect the two habitats had on uropod symmetry. These authors found statistically significant differences in uropods, with tube dwellers having symmetrical uropods while in shelldwelling individuals the uropods were asymmetrical. The influence of housing on uropod symmetry has been reported by several authors (see Imafuku & Ando 1999 for review), but Rodrigues et al. ’s (2002) study is the first to indicate that some examples of sexual dimorphism may be directly influenced by habitat. The domicile or domiciles of Catapaguropsis queenslandica n. sp. are not known; however, certain dimorphic attributes suggest that it or they are unlikely to be gastropod shells, and very possibly are different for the sexes.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |