Isoodon obesulus (Shaw, 1797)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6621742 |
|
DOI |
https://doi.org/10.5281/zenodo.6761998 |
|
persistent identifier |
https://treatment.plazi.org/id/03C91729-FFD7-FFB4-FD91-DD78F5BF1734 |
|
treatment provided by |
Felipe |
|
scientific name |
Isoodon obesulus |
| status |
|
1. View On
Southern Brown Bandicoot
French: Bandicoot obése / German: Kleiner Kurznasenbeutler / Spanish: Bandicut de hocico corto meridional
Other common names: Brown Bandicoot, Short-nosed Bandicoot, Southern Short-nosed Bandicoot; Nuyts Southern Brown Bandicoot (nauticus)
Taxonomy. Didelphis obesula Shaw, 1797 ,
“New Holland.” Restricted by T. Iredale & E. Le G. Troughton in 1934 to “Sydney, N.S.W. [= New South Wales],” Australia.
Further restricted by J. M. Dixon in 1981 to “1-5 km south of the Hawkesbury River, 7-5 km from the Coal and Candle Creek Road turnoff on the West Head Road, Ku-ring-gai Chase National Park, north of Sydney, New South Wales, Lat. 33° 36’ S; Long. 151° 16” E,” Australia.
This species has been split at various times into 3-5 geographically separated subspecies, but most contention surrounds the status of present species with respect to the closely related I. auratus . In some morphological and mtDNA analyses the latter has been grouped with Western Australian subspecies fusciventer, which would give this form a large and almost continuous historical distribution throughout Western Australia and most of the central part of the continent. More recent analyses incorporating additional nuclear-gene sequences suggest, however, that I. auratus is sufficiently distinct to warrant separation from I. obesulus , and this arrangement is followed here. Same analyses also hint at a closer relationship between the north-eastern subspecies peninsulae and I. auratus than between former taxon and other forms of I. obesulus , and show little divergence between nominate obesulus and Tasmanian race affinis . Nuyts Archipelago form nauticus is morphologically distinct from the nominate form, butis sometimes subsumed within latter. Pending further work, affinis, fusciventer, nauticus, and peninsulae are treated here as subspecies of 1. obesulus . Five subspecies recognized.
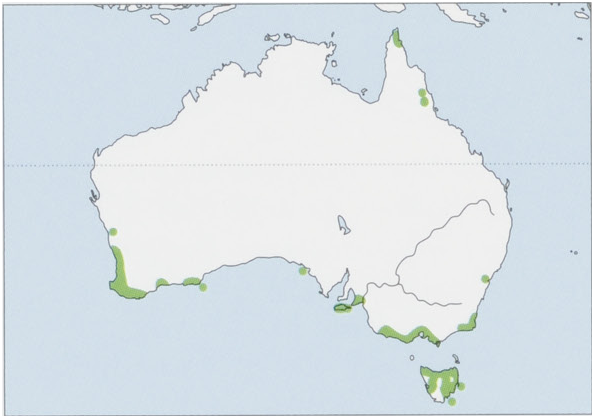
Subspecies and Distribution. I.o.obesulusShaw,1797—NewSouthWales,Victoria,mainlandSouthAustralia,andKangaroo I. I.o.affinisWaterhouse,1846—Tasmania. I.o.fusciventerGray,1841—WesternAustralia. I.o.nauticusThomas,1922—NuytsArchipelago,SouthAustralia. I. o. peninsulae Thomas, 1922 — N Queensland, including Cape York Peninsula. View Figure
Descriptive notes. Head-body 28-36 cm, tail 9-14.5 cm; weight 0.4-1.8 kg. Males up to 40% heavier than females. Dorsal fur is dark brown or dark gray, and has a grizzled appearance owing to presence of black spiny bristle hairs. Ventral furis light gray and softer than stiff dorsal hair, and often has a creamy or yellowish tinge. Tail is covered in short, dark hairs. Older animals may have a stubby tail, ragged ears, and patches of missing fur (from fighting).
Habitat. Diverse forest, woodland, heath and shrub communities, often where these occur on sandy soils. It occurs also on the edges of swamps and in riparian habitats where dense ground-level vegetation is present, creating tunnels and runs or using those constructed by other mammals. Recently burnt sites may be exploited for the regenerating food and shelter resources that they provide. Simple nests are formed under dense vegetation, under the skirts of grass trees (Xanthorrhoea spp., Xanthorrhoeaceae ), and occasionally in shallow burrows; plantlitter, soil, and other debris may be used as construction materials to help enclose the nest. This species used to occur in more open habitats, but is probably restricted to areas with dense ground cover that provide shelter from introduced predators such as the Red Fox (Vulpes vulpes) and the domestic cat (Felis catus).
Food and Feeding. Insects, small vertebrates (lizards, frogs, rodents), bird eggs, fruit, green plant material, and fungi are all eaten. In several studies invertebrates have been the dominant food item, but the relative importance of each food type varies with season and locality. Most foraging occurs at night, animals using their hearing to detect surface-active prey and their sense of smell to detect buried prey. Digs characteristic conical holes in topsoil when buried prey has been located, providing a ready and reliable means of determining whether the species is present at a site.
Breeding. Females attain sexual maturity at 4-5 months and males at six months. Breeding usually takes place from mid-winter to mid-summer, but can occur yearround. Gestation is c.14 days, and the 1-6 young (average 2-8) are weaned at 60 days. Females have eight nipples housed in a backward-facing pouch; different nipples are used by neonates of alternate litters, allowing time for recently used nipples to regress to a size that allows newly born young to attach to them. Recruitment of young into adult population is typically of the order of 5-15%.
Activity patterns. In the wild, usually nocturnal. Animals emerge an hour or more after dusk and appear to spend most of their active time in foraging. Captive individuals are strictly nocturnal and have been recorded as spending 71% of the 24hour day at rest in their nests.
Movements, Home range and Social organization. Like other peramelids, this species is solitary and intolerant of conspecifics except for brief encounters during courtship and copulation, and when females are with dependent young. Some evidence that males maintain territories incorporating resource-rich patches of habitat and tolerate presence of young females within them, but banish older females to poorer-quality habitats. Population densities range from 0-1 ind/ha in poor-quality habitat to 5 ind/ha in sites with abundant food and few predators; home ranges usually of the order of 1-2 ha, but may exceed 6 ha in areas with sparse resources. Although few young usually recruited to the adult population, this species’ high reproductive rate may allow it swiftly to colonize newly created habitats in the wake of wildfires and other disturbances.
Status and Conservation. Classified as Least Concern on The IUCN Red List. Shows a decreasing population trend. With the exception of races affinis and perhaps nauticus, the distributions of all subspecies have been fragmented and reduced in recent decades, this being especially marked in south-eastern Australia. The species occurs in conservation reserves throughout its range, but is susceptible to predation from introduced Red Fox and feral cat, and to loss of habitat in non-reserved areas. Nominate subspecies and Tasmanian endemic race affinis can be considered near threatened owing to depressive effects of predators and habitat loss or alteration, and Nuyts Archipelago form nauticus is vulnerable owing to the small size and restricted area of its population. Cape York subspecies peninsulae has small area of occupancy, but is abundant within its small range and considered to be of least concern. Likewise, race fusciventer in Western Australia is considered to be of least concern; it has declined in the past, but has achieved partial recovery since mid-1990s owing to large-scale and highly successful campaigns to reduce Red Fox numbers. Programs to reduce impacts of introduced predators are currently underway also in Victoria, South Australia, and New South Wales, and these should help to guard the security of the species in future.
Bibliography. Ashby et al. (1990), Broughton & Dickman (1991), Brown & Main (2012), Close et al. (1990), Coates & Wright (2003), Coates et al. (2008), Cockburn (1990), Copley et al. (1990), Dexter & Murray (2009), Dickman (1988b, 2012, 2014), Dixon (1981), Friend, J.A. (1990a), Gordon etal. (1990), Green (2007), Groves (2005c), Haby, Conran & Carthew (2013), Heinsohn (1966), Hocking (1990), Hope (2012), Iredale & Troughton (1934), Keiper & Johnson (2004), Kemper (1990), Larcombe (2003), Li You et al. (2014), Lobert (1990), Lobert & Lee (1990), Lyne & Mort (1981), McKenzie (1967), Menkhorst & Seebeck (1990), Morris et al. (1998), Nagy et al. (1991), Pope et al. (2001), Quin (1988), Stoddart & Braithwaite (1979), Thomas (1990), Tyndale-Biscoe & Renfree (1987), Warburton, Grégoire et al. (2013), Westerman et al. (2012), Woinarski et al. (2014e, 2014f, 2014g, 2014h), Zenger et al. (2005).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Metatheria |
|
Order |
|
|
Family |
|
|
Genus |