Liassorhyphus liaoi Nel & Huang, 2022
|
publication ID |
https://doi.org/ 10.11646/palaeoentomology.5.2.12 |
|
publication LSID |
lsid:zoobank.org:pub:D5677074-3D13-4D8A-8B9C-03BE43E1DA47 |
|
DOI |
https://doi.org/10.5281/zenodo.6532875 |
|
persistent identifier |
https://treatment.plazi.org/id/83AFEC0D-75BE-419B-8E48-A3CE1E0253DC |
|
taxon LSID |
lsid:zoobank.org:act:83AFEC0D-75BE-419B-8E48-A3CE1E0253DC |
|
treatment provided by |
Plazi |
|
scientific name |
Liassorhyphus liaoi Nel & Huang |
| status |
sp. nov. |
Liassorhyphus liaoi Nel & Huang , sp. nov.
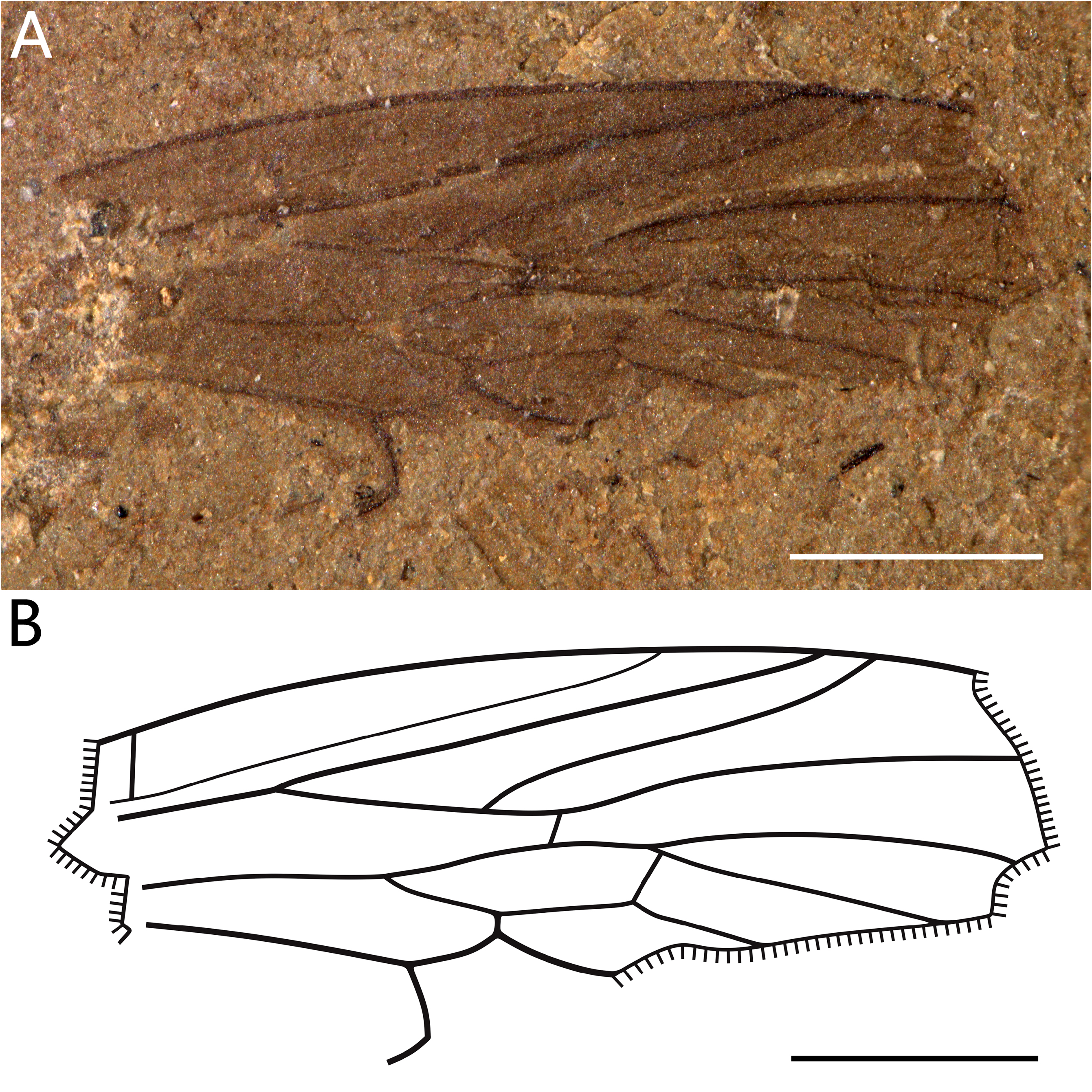
( Fig. 5 View FIGURE 5 ) urn:lsid:zoobank.org:act:83AFEC0D-75BE-419B-8E48-A3CE1E0253DC
Material. Holotype ( NIGP180152 View Materials ), only one specimen, incomplete, slightly deformed.
Etymology. Named after Professor Zhuoting Liao, a well-known palaeontologist for Late Palaeozoic brachiopods and stratigraphy, the guider of the field trip of this study at 2018. He passed away during the field work in the Guangxi Zhuang Autonomous Region in 2019 at the age of 80.
Diagnosis. As for genus.
Type locality and horizon. A fossil locality near the Daheyan Town, Turpan City, Xinjiang; late Early Jurassic .
Description. Coloration not preserved; wing ca. 4.0 mm long and ca. 1.4 mm wide; Sc ≥ 0.5× wing length, ending on anterior margin of wing 0.6 mm distad level of base of R 2+3; three radial branches; vein R 4 fused into R 4+5; R 4+5 elongate (longer than half wing-length); crossvein rm between R 4+5 and upper margin of d-cell, as R 4 and R 5 fused in R 4+5; four medial branches; M 1 and M 2 leaving d-cell with a short petiole; M 1 and M 2 shorter than half of wing length; CuA strongly arched posteriorly in its distal part; crossvein cua-m very long and curved; d-cell closed, 1.0 mm long, longer than 0.1 wing length, located near mid wing; R 1 and R 2+3 subparallel; apices of R 1 and R 2+3 weakly approximate; R 2+4 slightly arched; stem of M well-developed; R 4+5 arched; alula not preserved.
Remarks. The Anisopodoidea is a very ancient ‘nematoceran’ fly superfamily, currently comprising the two extinct Protorhyphidae Handlirsch, 1906 and Siberhyphidae Kovalev, 1985 , and the fossil and extant Anisopodidae Knab, 1912 . Some of the oldest known Diptera belong to this superfamily (e. g., Vymrhyphus blagoderovi Krzemiński & Krzemińska, 2003 ). Wojtoń et al. (2018) listed the fossil genera and species and proposed a phylogenetic analysis of the group, using Plecia americana (Bibionidae) as outgroup in their analysis. The superfamily Anisopodoidea is currently considered as a member of the infraorder Bibionomorpha Hennig, 1954 , but Zhang X. et al. (2022) proposed, after a molecular phylogenetic analysis that the extant Anisopodidae would be the sister group to the Brachycera, the clade Bibionomorpha being the sister group to the( Anisopodidae + Brachycera). If confirmed, this position would suggest that it is necessary to re-make the phylogenetic analysis of the Anisopodoidea with the addition of some Brachycera as outgroups to improve the polarization of the characters in the analysis of Wojtoń et al. (2018).
Nevertheless, as it is, the work of Wojtoń et al. (2018) is very useful to determine the positions of new taxa in the Anisopodoidea . This superfamily, being in a ‘link’ position between ‘Nematocera’ and Brachycera, is of major importance to better understand the evolution of the flies. With only five extant genera vs. 15 strictly Mesozoic genera, the Anisopodoidea were clearly more diverse at that time than now a day.
This fossil wing is very similar to those of the extant Anisopodidae (e. g., Sylvicola Harris, 1780 or Olbiogaster Osten Sacken, 1886 ), especially in the simple R 2+3 and R 4+5, the shape of the d-cell and of the branches of M. After the phylogenetic analysis of Wojtoń et al. (2018), affinities of the new fossil with the Protorhyphidae (a Triassic–Jurassic group) are excluded because of the following characters: three radial cells in the new fossil (vs. four); vein R 4 fused into R 4+5 (vs. R 4 well-developed); r-m between R 4+5 and upper margin of d-cell (a rather weak character because, in Protorhyphidae , it is between R 4+5 stem and d-cell, because there is a stem of R 4+5 as R 4 and R 5 are distally separated); R 4+5 elongate (same situation as before because, in Protorhyphidae , R 4 and R 5 are separated). Affinities with the clade ( Mesochria + Mycetobia ) ( Mycetobiinae , a clade with a fossil record going into the mid-Cretaceous; Szadziewski et al., 2016; Kania et al., 2019a; Camier & Nel, 2019) are excluded because the new fossil has four branches of M, the d-cell is opened, and there is a long petiole of M 1 and M 2. The new fossil shares with the two taxa Vymrhyphus blagoderovi Krzemiński & Krzemińska, 2003 (in Protorhyphidae , thus with affinities excluded; Krzemiński & Krzemińska, 2003) and Megarhyphus sophiae Kovalev, 1990 , the short petiole of M 1 and M 2 ( Kovalev, 1990; Zhang, 2007). The other anisopodoid taxa studied by Wojtoń et al. (2018), including Megarhyphus amberae Krzemińska et al., 2010 , have the veins M 1 and M 2 leaving d-cell independently. Megarhyphus rarus Zhang, 2007 has M 1 and M 2 emerging at the same point. The three Megarhyphus spp. (Late Jurassic–Early Cretaceous) share with the new fossil a large d-cell situated in the mid part of wing, but all differ from the new fossil in R 1 and R 2+3 strongly approximating at wing margin, a m-cu shorter than in the new fossil, and a distal part of CuA smoothly undulating, vs. strongly curved posteriorly in the new fossil ( Zhang, 2007; Krzemińska et al., 2010).
De Souza Amorim & Tozoni (1994) separated the Olbiogastridae (Olbiogastrinae in Wojtoń et al., 2018) from the other Anisopodoidea on the basis of the character ‘Cell m1 tapering proximally/ cell m1 with an angle proximally’, corresponding to differences in the bases of veins M 1 and M 2 ( Pratt & Pratt, 1980). But this character [‘presence of a short petiole of M 1 and M 2 ’ & ‘M 1 and M 2 emerging at the same point’ & ‘M 1 and M 2 emerging independently’] appears not sufficient to clearly separate the genera, as the situation may vary within the same genus (e. g., Megarhyphus and Sylvicola ). Besides, Wojtoń et al. (2018) did not included several key genera in their work.
As such, we need to search for other arguments to separate the new fossil from the various anisopodid genera. Tega Blagoderov et al., 1993 (Teginae Lukashevich, 2012, Middle –Late Jurassic) has a very small d-cell, located in basal half of wing, unlike the new fossil. Lukashevich (2012: 281) indicated about the Cretaceous genus Thiras Giebel, 1856 that ‘Since the holotype of Thiras westwoodi Giebel, 1856 has not yet been re-examined, it remains unclear whether Pachyrhyphus is a junior synonym of Thiras Giebel, 1856 ’. Westwood (1854: pl. 18, fig. 20) figured the wing of Thiras westwoodi ; it clearly has an opened d-cell. The late Jurassic–Early Cretaceous genus Pachyrhyphus Kovalev, 1986 has veins R 1 and R 2+3 strongly approximating at wing margin, a closed d-cell and a vein M 1 emerging from anterior side of this cell, unlike the new fossil ( Kovalev, 1986, 1990). The genera Olbiogaster, Eogaster De Souza Amorim & Tozoni, 1994 , and Australogaster De Souza Amorim & Tozoni, 1994 have the veins R 1 and R 2+3 strongly approximating at wing margin, unlike the new fossil ( Edwards, 1915; Tonnoir, 1923; Okada, 1938; Tozoni, 1993; Grimaldi & De Souza Amorim, 1995).
The Jurassic Mesorhyphus Handlirsch, 1920 has the apex of Sc situated at level (or nearly so) of base of R 2+3, while it is much longer in the new fossil, and its R 2+3 is much shorter than in the new fossil ( Ansorge & Krzemiński, 1995; Lukashevich, 2012). Cretolbia Kania et al., 2019 (mid-Cretaceous) and the extant genus Carreraia Corrêa, 1947 have also the apex of Sc situated at the level of base of R 2+3 ( Corrêa, 1947: fig. 1; Kania et al., 2019b). Sylvicola (Cenozoic to extant) has the vein R 2+3 strongly sigmoidal, unlike the new fossil ( Krivosheina & Menzel, 1998; Wojtoń et al., 2018). The species of the (sub-)genus Anisopus Meigen, 1803 have the veins M 1 and M 2 leaving d-cell independently or at the same point ( Fuller, 1935; Lane & d’Andretta, 1958; Pratt & Pratt, 1980). The Middle Jurassic genus Leptoplecia Hong, 1983 is based on an incomplete specimen with the wing venation poorly preserved ( Hong, 1983: text-fig. 113, pl. 27, fig. 4), hardly comparable with the new fossil. The Middle Jurassic genera Jurolaemargus Evenhuis, 1994 (type species Jurolaemargus yujiagouensis ( Hong, 1983) and Gansuplecia Hong & Wang, 1990 are supposed to have a forked R 4+5 and an opened d-cell ( Hong, 1983: text-fig. 112; Hong & Wang, 1990: text-figs 140–141). The Siberhyphidae Kovalev, 1985 ( Siberhyphus lebedevi Kovalev in Kalugina & Kovalev, 1985, Middle Jurassic ) have no R 2+3 ( Kovalev, 1985: text-fig. 65). Lobogaster Philippi, 1865 has a sinuous and shortened R 4+5 ( De Souza Amorim & Tozoni, 1994).
In conclusion, the new fossil does not fit in any of the described genera of Anisopodoidea . Its most remarkable characters are ‘CuA strongly curved posteriorly’ and ‘crossvein cua-m very long and curved’, allowing to separate it from all the anisopodoid genera.
The discovery of Liassorhyphus liassicus Nel & Huang , gen. et sp. nov. confirms the diversity of the Anisopodoidea in the Liassic. Together with Megarhyphus amberae from the Liassic of UK (196.5–189.6 Ma), it is the second earliest Jurassic record of the Anisopodidae , while the other Liassic representatives of the superfamily belong to the Protorhyphidae ( Protorhyphus ovisimilis Bode, 1953 , Protorhyphus simplex (Geinitz, 1888) , Protorhyphus stigmaticus Handlirsch, 1920 , Protorhyphus turanicus Rohdendorf, 1964 , Archirhyphus geinitzi Handlirsch, 1939 , and Austrorhyphus moryi Martin, 2008 ). The whole group had been already widely distributed at that time ( Australia, Europe, Central Asia), suggesting even it had a higher diversity than what is known after its fossil record.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
InfraClass |
Lower |
|
SuperOrder |
Odonatoptera |
|
Order |
|
|
SubOrder |
Nematocera |
|
InfraOrder |
Bibionomorpha |
|
SuperFamily |
Anisopodoidea |
|
Family |
|
|
Genus |