Austromartyria Gibbs
|
publication ID |
https://doi.org/ 10.5281/zenodo.196244 |
|
DOI |
https://doi.org/10.5281/zenodo.6195752 |
|
persistent identifier |
https://treatment.plazi.org/id/03CD296B-E40A-E174-4FA7-5304F680FE48 |
|
treatment provided by |
Plazi |
|
scientific name |
Austromartyria Gibbs |
| status |
|
Austromartyria Gibbs View in CoL , gen. nov.
Type species: Sabatinca porphyrodes Turner, 1932 , by present designation.
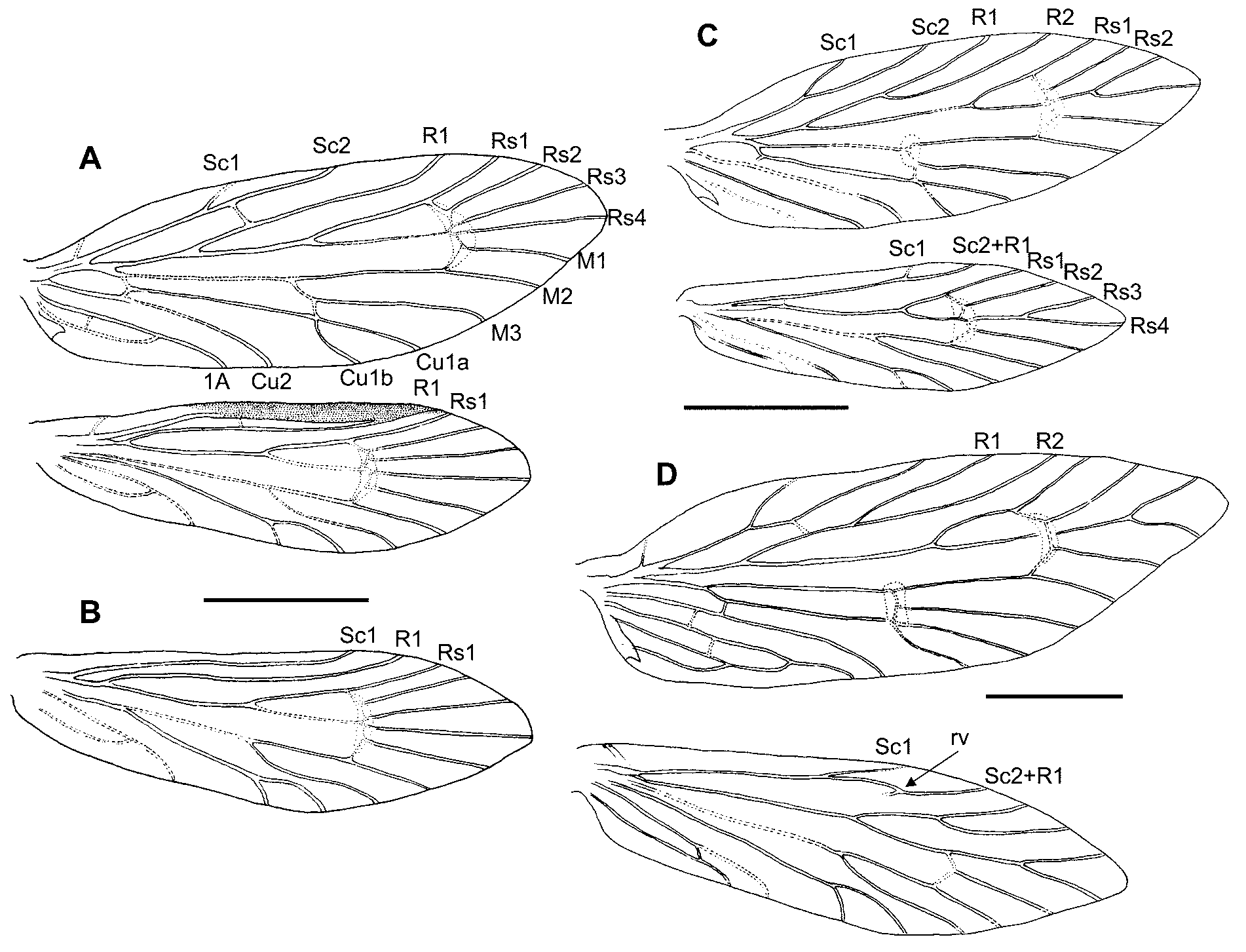
Diagnosis. The combination of a simple (unforked) forewing vein R1 and a separate hindwing R1 ( Figs. 4 View FIGURE 4 A & B) is unique in the family, with the exception of the (otherwise very different) northern hemisphere genus Micropterix . As a monotypic genus, the character—male with thickened glandular costal margin on hindwing —is, for all practical purposes, diagnostic ( Figs. 4 View FIGURE 4 A & 6A).
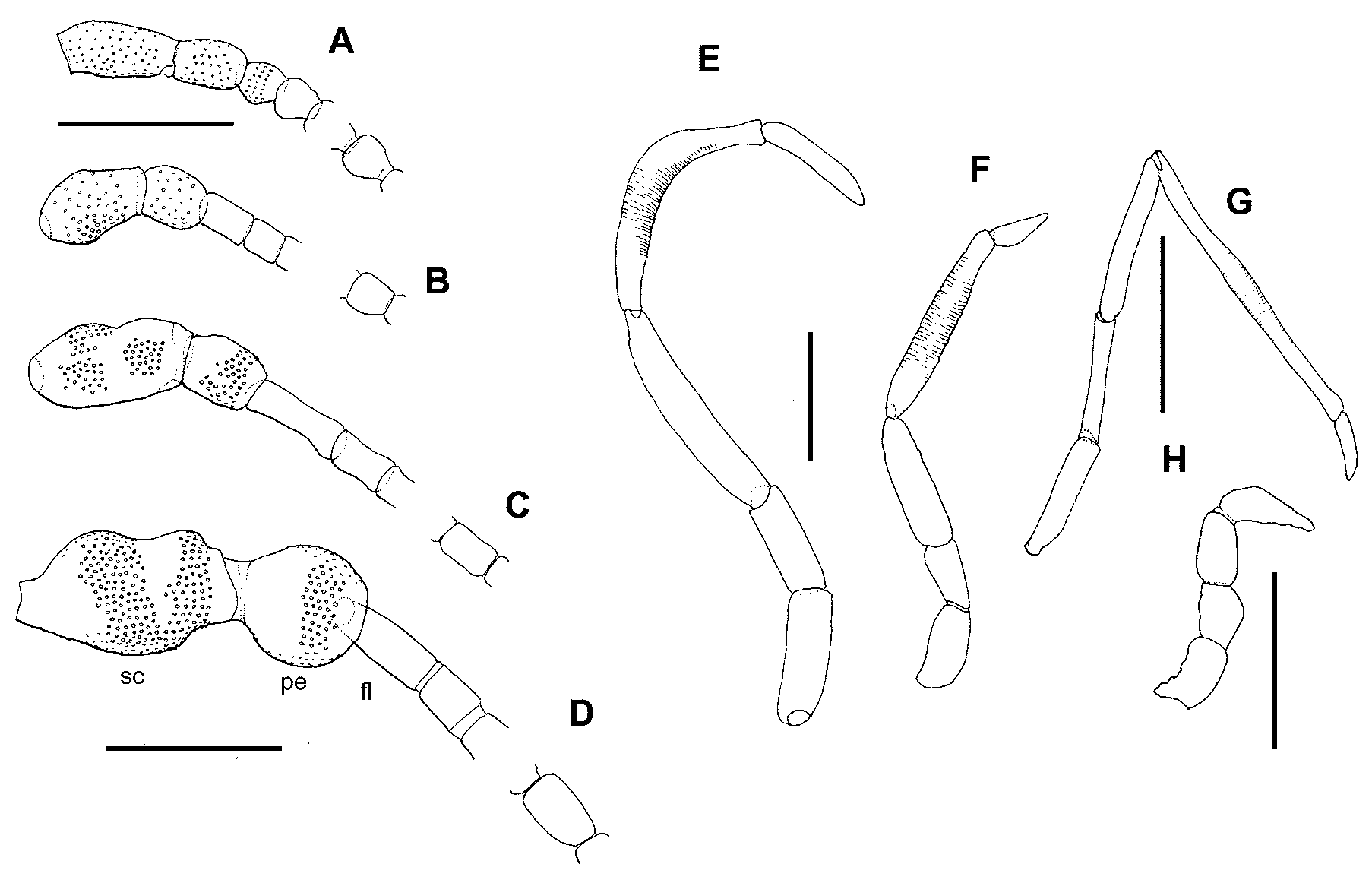
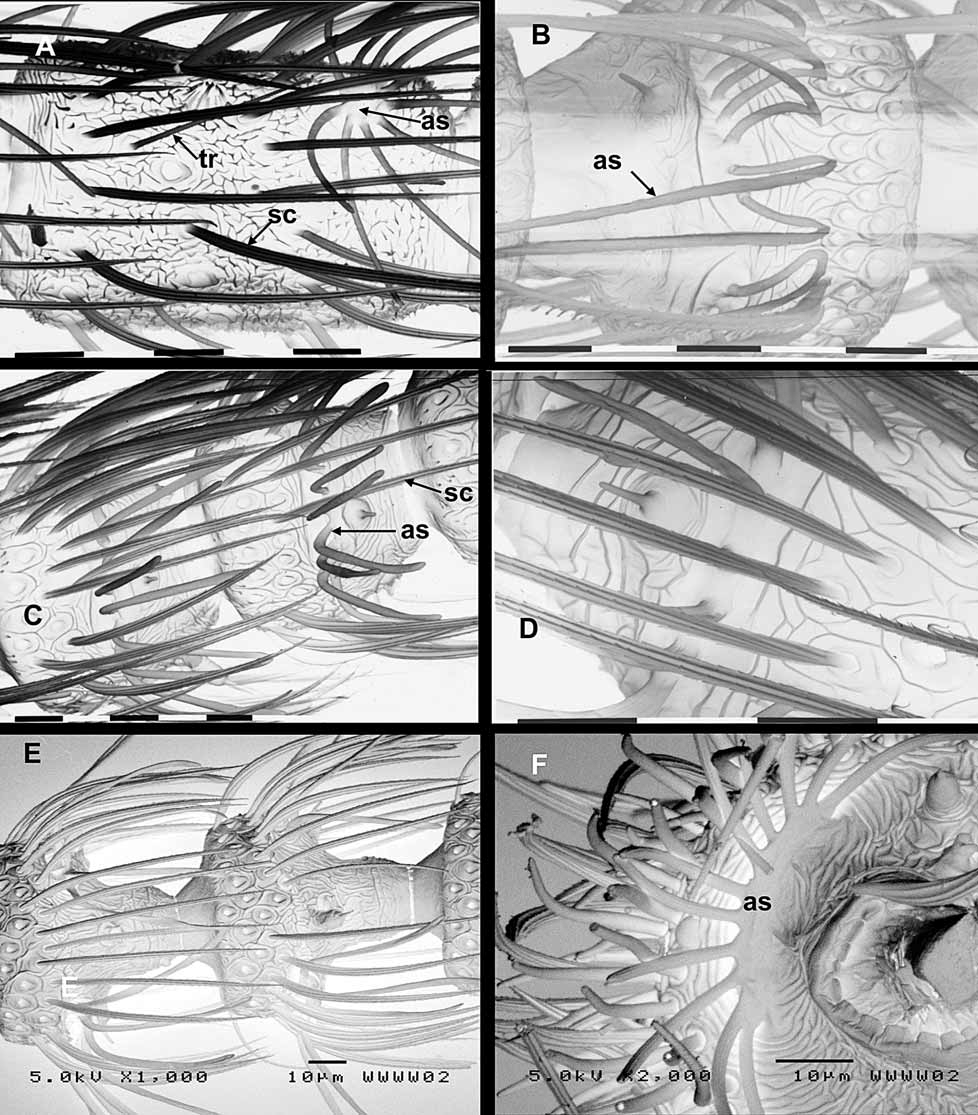
Description. Head interocular index ( Davis 1975) 0.8 in both sexes. Ocelli present. Vertex between ocellus and compound eye microtrichiated. Head clothed with tufts of long piliform scales. Antennal scape swollen and barrel-shaped with a shallow indentation at mid-length but otherwise not markedly dumbbellshaped; pedicel swollen, equidimensional (ratios of scape:pedicel:first flagellomere are length = 2.5:1.9:1, and width = 1.5:0.8:1 ( Fig. 3 View FIGURE 3 C). Flagellum in male of 43–45 flagellomeres, densely covered with lamellar scales on 3–4 basal flagellomeres; female antenna shorter, 33–34 flagellomeres with basal 5–6 lamellarscaled. Flagellomeres filiform (elongate cylindrical), paired antennal ascoids present towards the distal end of each flagellomere beyond the lamellar scale-covered ones, each with a circular discoid base from which 7–10 long radiating branches extend, the branches more closely spaced around the base on the distal than proximal side; surface of flagellomeres with a reticulate pattern of fine wrinkles; flagellomeres sparsely clothed with long narrow fluted scales and the occasional long trichoid sensilla ( Fig. 5 View FIGURE 5 A). Mandibles robust, slightly asymmetrical, bearing numerous small blunt teeth along inner margins with a single larger tooth at apex of right mandible; close-set hairs on the proximal inner angles. Labial palps 2-segmented. Maxillary palps 5- segmented, 1.6x head width; ratio 1:0.7:1.7:1.7:0.9; segment 4 crescent-shaped, swollen in middle region, with fine transverse striations almost throughout ( Fig. 3 View FIGURE 3 E).
Thoracic tegulae with a tuft of long piliform scales. Foretibial epiphysis well developed.
Wing venation as shown in Figs. 4 View FIGURE 4 A, B. Forewing with unpigmented patch extending around bases of Rs1 to M3 and another patch around primary M fork; Sc forked; R1 unforked; sc-r crossvein reaching R beyond fork; Rs veins all ‘sessile’; Rs4 to apex. Hindwing with Sc and R1 separate and parallel from base with cross vein sc-r at mid-length, both veins running parallel to costa in female but coalescing toward costa in male; crossvein inter-Rs unmelanised; Rs3 and Rs4 free; Rs4 to apex; 1A+2A forming a loop to CuP but not reaching to anal margin; 2–3 frenular bristles. Sexual dimorphism evident along costal area of both fore-and hindwing; the male wing being thickened with a conspicuously specialised (surely glandular) zone, staining strongly with acid fuchsin; characterised on both upper and lower surfaces by numerous circular setal bases from each of which a very slender seta arises, the whole area being overlain with linear scales ( Fig. 6 View FIGURE 6 A). The presumed glandular zone is narrow and inconspicuous along the forewing costa but on the hindwing comprises a broad melanized bar between the costal margin and Sc, extending from 37% to 80% of the wing length, and distorting the path of Sc so that it coalesces with R1 at the margin ( Fig. 4 View FIGURE 4 A). So far as is known, this glandular development is unique in the family.
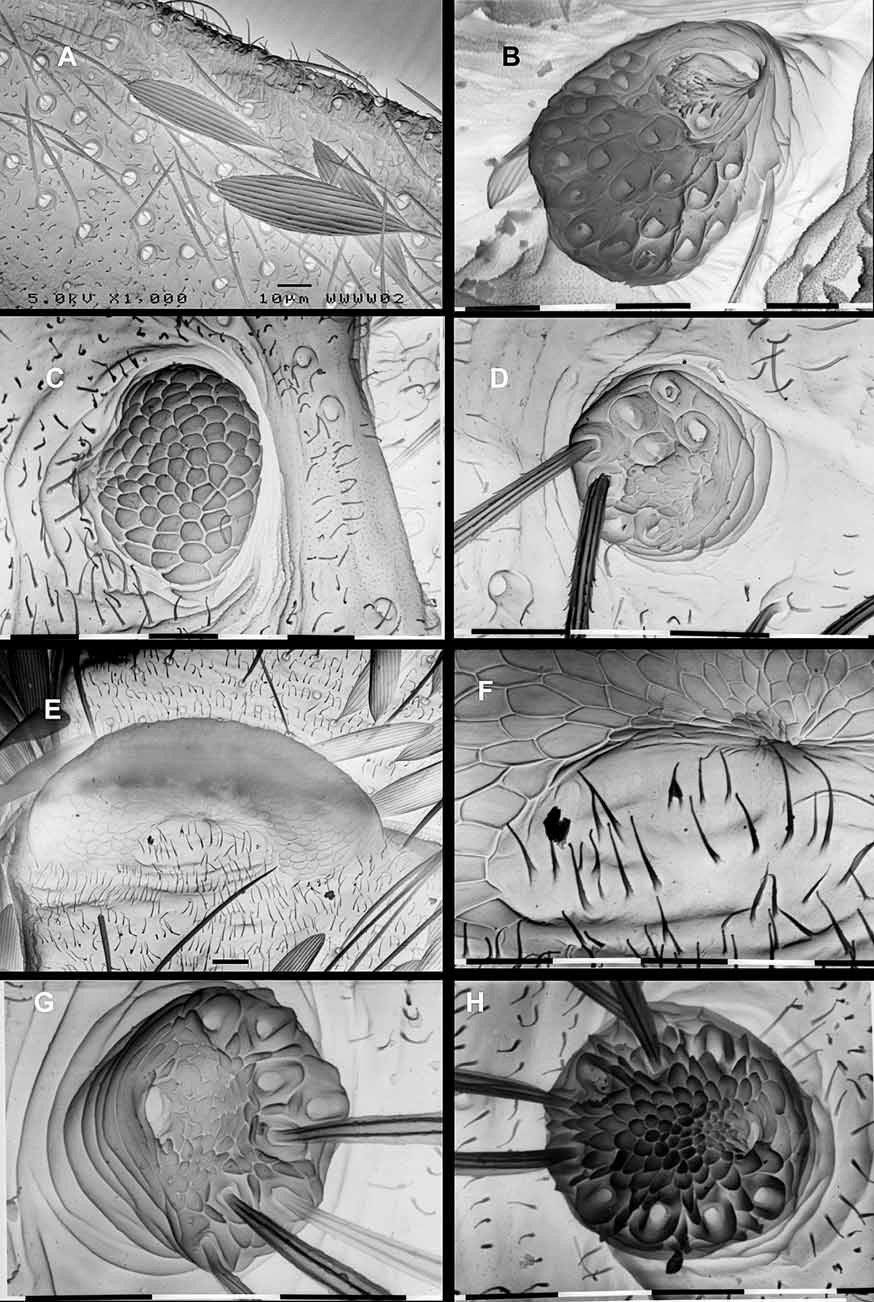
Male abdomen and genitalia [G894]. (Figs. 8A, C–H) Dorsum of A1 with two transverse bands of sparse scales across the membrane and a very weak transverse sclerite in the male only; anterior rim of T1 entire, posterior rim interrupted in middle. Male abdomen uniquely modified into a distinctive “clasping type, with some overall resemblance to the form seen in Micropterix ; narrowed, and strongly arched upwards posteriorly (Fig. 8A); a weak dorsal median keel on T2, keel becoming progressively pronounced posteriorly to be strongly developed on T6, T7 and T8; sternites reduced to elongate sclerites, becoming progressively narrower towards posterior, where they are about twice as long as wide, tapering slightly posteriorly (Fig. 8A). Female abdominal tergites and sternites normal. S5 gland present in both sexes ( Fig. 6 View FIGURE 6 B), its exit area protuberance in the male situated on extreme anterior-lateral angles of the “T-shaped sternite (Fig. 8B), in female close to anterior margin; raised on a short peduncle and bearing about 17 (female) to 24 (male) very long piliform-scales; aperture crescent-shaped, on anterior edge, with a field of minute cuticular dendrites between aperture and hair-scale bases; these bases uniformly distributed over most of the distal surface of the gland ( Fig 6 View FIGURE 6 B).
A discrete, elongate, unadorned tergum 10 projects up at a 45 angle, flanked by two slightly divergent gonopods (valvae) with dense mesial brushes. Segment A8 with no trace of independent sternal sclerotizations. Segment 9 sclerite very short, especially in relation to other sabatincoid taxa, 0.3x S6; its dorsal arms not meeting in mid-line; anteroventral margin not extended forward, in repose not reaching to S7; the long antero-lateral margin moderately thickened and melanized over most of its length; posterior margin convex. Valvae divergent, simple, broadly triangular with ventral edge almost straight, dorsal edge strongly arched; inner face slightly concave; a dense brush of long soft bristles arising from the inner face along the posterodorsal slope, curving towards centre line; valve bases more or less fused mid-ventrally, not articulated from within posterior margin of S9. Median plate a narrow vertical flange, fanning out anteriorly (in lateral view); nearly as long as ventral margin of S9 (0.9) but posterior end arising within valva base. Tergum 10 straight, elongate, about 3x longer than wide; 3.4x ventral length of S9; tapering slightly to a bluntly rounded posterior apex with a minute indentation in the mid-line; completely separated from sclerite 9; upper surface melanized, convex, bearing scattered fine setae; undersurface membraneous, without setae. Anal cone very prominent, strengthened by a pair of non-setose lateral melanized sclerites, separate from T10, elongate triangular, tapering posteriorly and curved around the anal cone, densely clothed with acutely pointed microtrichia; attributed to venter 10 ( Kristensen 1984a). Phallus (Fig. 8F–H) short in relation to that of Sabatinca s.str., 1.3x S6. Distal part of phallus shorter than phallobase (0.5); a short tapering ventral bulb curves upwards beyond the gonopore; between the apex of the ventral bulb and the gonopore is a thin-walled sac-like structure extended laterally on each side at the level of the gonopore and reflected ventrally to form a pair of lightly melanized lappets level with the proximal edge of gonopore; gonopore small, transverse, crescent-shaped, oriented dorsally and forming a slight prominence in lateral view; bordered by sclerotised radial folds. Phallocrypt clothed with large, acutely pointed micro-scales.
Female genitalia. (Figs. 8I, J) Segment 9 sclerite forming a continuous ring, about twice as high as long; set with scattered, simple setae; dorsal length equal to ventral length. Lateral sclerites of paired ‘terminal papillae’ (assumed to be attributable to Segment 10) roughly equidimensional, almost circular in outline; set with a regular array of stiff setae. Genital chamber with a short thickened ‘papilla’ encircling the base of the ductus spermathecae, archway-shaped, orientated anteriorly in relation to insertion of the ductus; contiguous ventrally with a short thickened region over the entrance to the bursa. Spermatheca relatively short, of three distinct sections; a short very thick-walled basal duct, slightly sinuous, about the same length as the height of the ‘papilla’, leading into a slightly expanded thin-walled bulb; a short extremely narrow section connecting this ductus section to the base of utriculus, with its walls minutely papillated; an elongate (4.5x longer than wide) thin-walled utriculus, a parallel-sided cylinder with irregularly spaced septum-like constrictions, utriculus tapering abruptly at distal end where the lagena is inserted; lagena narrow, tubular, loculate, about two thirds the length of utriculus and terminating in a short filament. Corpus bursae small, simple, lacking any sclerotised adornment.
Larva. ( Figs. 7 View FIGURE 7 A–F) Three small micropterigid larvae (second instar?), collected from within the known distribution of this species; two at The Crater, Hypipamee National Park and one at Henrietta Creek, Palmerston National Park, North Queensland, have been putatively assigned to this taxon. They were obtained by heat extraction from the periphyton layer on rotten logs. These Australian larvae are clearly distinct from the larval type attributed to Tasmantrix (see below).
The larvae are typically ‘sabatincoid’ in form, i.e. with eight pairs of abdominal spiracles; the trunk broadly hexagonal in cross-section, and raised along dorsum between mesothorax (T2) and abdominal segment 8 (A8) with squarish dorsal ridges from which D1 setae arise; integument with a honeycomb of raised ridges, a light greenish pigmentation in life with darker inter-segmental patches.
Head ( Figs 7 View FIGURE 7 D,E) capable of being totally recessed into the prothorax. Cranial ecdysial lines not visible on the immature specimens available; a cluster of 5 stemmata on each side within a melanised ‘cage’; hypostomal bridge without antero-medial melanisation. Chaetotaxy typical of sabatincoid larvae ( Davis, 1987, Hashimoto, 2001) with all setae and microsetae represented. The unique micropterigid median seta M1 on frontoclypeus is situated between the antennae, sufficiently far forward that all five setae (including paired M1 and the shorter AF1) are approximately along one transverse line ( Fig 7 View FIGURE 7 E).
Thoracic legs ( Fig. 7 View FIGURE 7 F) short, three-segmented, lacking a distinct melanised coxa; femur with an inflatable membraneous sac on its inner side near the base, and bearing three blunt peg-like setae toward distal end, two microsetae around basal margin; tibiotarsus with three very short peg-like setae, tarsal claw well developed with a small seta at its base.
Abdominal segments 1–8 with ‘incipient’ prolegs, short projections with rounded apices. Functional spiracles present on abdominal segments 1–8; conspicuous, raised and melanised; placed in the middle of each segment, above the lateral ridge (and hence L1) on A2–8 but below this ridge on A1. Trunk ( Fig 7 View FIGURE 7 A,B) dominated by strong dorsal and lateral longitudinal ridges, the D1 setae set along the dorsal ridge and L1 along lateral ridge; neither with enlarged raised bases typical of many sabatincoid larvae, except at ends of the trunk on T1 & T2 and A8 & A9. Between T2 and A8 the longitudinal dorsal ridges are elevated, raising the profile of this region substantially above the line of the prothorax and segment A9. In this region the middorsal muscle attachment sclerites (platelets) are evident.
Setae of dorsal (D), lateral (L) and subventral (SV) areas bluntly clubbed (clavate) with a slightly irregular apex, the majority short to extremely short (here referred to as ‘spherical microsetae’ where reduced to a minute spherical bulb), but a few moderately long setae on dorsum at the anterior and posterior extremities of the trunk ( Fig 7 View FIGURE 7 C). Short unmelanised setae of normal form with an acute apex are referred to here as minisetae. Note that the SD setae are considered to be absent from all micropterigid larvae ( Hashimoto 2006).
Prothorax with 10 pairs of major setae, all directed forward, five of them aligned around the margin of the head recess fold; plus a minute spherical V1 seta on mesal side of coxa. On dorsum, five pairs on raised integumental bases, with two forming a longitudinal row either side of mid-dorsal line (here designated D1 & D2, with D2 on the anterior head recess margin), and three forming a discrete oblique row along a dorsolateral ridge (two most anterior here designated as the XD setae, the most posterior as L1), the remaining two L setae are aligned vertically at the level of the spiracle (here L2 & L3) close to the margin of the head recess, both clubbed, the central one slightly shorter; two SV setae in a horizontal row above the coxal area, SV1 a spherical microseta more or less dorsal to the coxa, SV2 a mini-seta on the margin of the head recess; MV1 a single mini-seta on the head recess margin toward mid-ventral line. Meso- and meta-thorax each with six pairs of clubbed setae, around the middle of each segment, distinguishable by their lengths, those of D and L groups being longer on T2 than on T3; the D1 seta of T2, second longest of the trunk, is inclined forward whereas on T3 is extremely short; D2 a spherical microseta, but slightly extended into a cup-shaped form on T2; two L setae, L1 the major one situated on the crest of the lateral ridge, on T 2 in vertical line, L2 below being one third length of L1, on T3 the L setae lie horizontally along the lateral ridge, L2 a spherical microseta; two SV setae in horizontal line above coxa, the more anterior being a microseta; V1 a spherical micro-seta on mesal side of coxa.
Abdominal segments each with five pairs of clubbed setae, two D setae, two L setae and a single SV seta. On A1–7 the setae are very reduced in size with D1 and L1 extremely short and clubbed, only slightly longer than wide, the others microsetae; on A8, D1 is the longest seta on the trunk, on A9 it is about two-thirds shorter; L1 slightly longer on A8 and A9 than on remainder of abdomen. Segment A9 with three pairs of setae, SV a microseta; A10 with two pairs, both microsetae.
Comparative remarks. Austromartyria displays a mixture of features that in sum are ‘sabatincoid’ but require re-appraisal of what is meant by that term. The forewing venation is unique in the southern hemisphere with its unforked R1 vein, although all northern genera show this condition. However, retention of the entire R1 vein in the hindwing is reminiscent of other ‘relic Sabatinca ’ micropterigid taxa in the southern hemisphere – Hypomartyria and three new genera from South Africa and Madagascar (N.P. Kristensen, pers. comm.) – as well as some New Zealand species of Sabatinca . Interpretation of these veins is complicated by the presence of sexual dimorphism in Austromartyria , associated with the glandular costa of the male. The unbranched hindwing R1 vein is entirely separate from Sc in female but coalesces towards the costa in the male (which also shows a trace of a cross vein at mid-length. The phylogenetic significance of these hindwing variations still remains an open question ( Kristensen & Nielsen 1982). Although specialised sexual dimorphism in the wings of A. porphyrodes and the highly modified male abdomen represent the most obvious autapomorphies for Austromartyria , I hesitate to stress these characters because in a more speciesrich genus they could well prove to be of species-level importance only.
The male genitalia, while contrasting with Sabatinca s.str. illustrate several parallels with the undescribed Madagascan genera. These include the short 9th sclerite, the elongate median tergum 10, and a very prominent anal cone with lateral sclerites. Although the elongate median T10 appears strikingly different from the bilobed tergum of typical sabatincoid taxa, it should be noted that New Zealand Sabatinca s.lat. species illustrate the entire range from a deeply bilobed tergite (e.g. S. lucilia ), through a simple median plate (e.g. ‘Sabatinca’ chrysargyra) to an extremely elongate median tergite (e.g. Micropardalis aurella ), more extreme than in A. porphyrodes . In contrast to the foregoing southern hemisphere characteristics, the type of phallus, with its short ventral branch (ventral bulb) extending beyond the gonopore, is a feature otherwise only known from the Northern Hemisphere micropterigids, Micropterix , Epimartyria ( Kristensen 1984a) and Vietomartyria ( Hashimoto & Mey 2000) .
Although larval characters are still unknown for a number of southern micropterigid genera, it is not entirely premature to examine whether these larvae shed any light on the question of wider southern relationships. In the case of Austromartyria , a preliminary larval character analysis (Gibbs unpublished data) suggests a sister grouping with Hypomartyria ( Chile) . Most striking is the sharing of the unique alignment of abdominal spiracles by these two genera, in which the spiracle on A1 lies below the longitudinal line of the remaining seven spiracles. They also share (including Agrionympha ) the vertical alignment of L2 and L3 on the prothorax. Although more rigorous analyses are still needed, it is becoming increasingly likely from molecular data, adult morphology (wing venation) and the larval features, that Austromartyria is indeed a member of the ‘southern sabatincoid’ lineage that includes Agrionympha and Hypomartyria , and very possibly other undescribed afrotropical and neotropical taxa ( South Africa, Madagascar, Costa Rica) (Davis, Gibbs, Kristensen & Lees, unpublished ms).
Etymology. The generic name is derived from the Latin australis (southern) with reference to the location of this monotypic sabatincoid genus, the sole Australian representative of a micropterigid lineage referred to loosely as the ‘relic Sabatinca group’ which occur around the southern hemisphere. The ending -martyria signifies its geographic equivalence to Hypomartyria from Chile.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.