Tubulipora patina Lamarck, 1816: 163
|
publication ID |
https://doi.org/10.1206/0003-0090(2002)270<0001:NABFTV>2.0.CO;2 |
|
persistent identifier |
https://treatment.plazi.org/id/03D1878C-1925-FFDB-FF90-C6CAFC13C4CF |
|
treatment provided by |
Felipe |
|
scientific name |
Tubulipora patina Lamarck, 1816: 163 |
| status |
|
Tubulipora patina Lamarck, 1816: 163 . Diastopora patina: Hincks, 1880: 458 . Berenicea patina: Marcus, 1940: 73 . Plagioecia patina: Canu, 1918: 327 . Harmelin,
1976: 129. Hayward and Ryland, 1985a: 97. Diastopora simplex Busk,1875: 28 . Diastopora latomarginata: Waters, 1879: 272 .
Friedl, 1917: 277. Discosparsa annularis Heller, 1867: 123.
DESCRIPTION (AMNH 947, 1030 –1032; CMRR 2276): Colonies white, multiserial, discoidal, with basal wall extended as broad lamina (up to 1 mm) beyond colonymargin zooidal buds. Colonies typically several millimeters, occasionally over 1 cm, in diameter; adherent to the substratum or with elevated perimeter. One or more generations of secondary, ‘‘daughter’’ colonies commonly budded peripherally on large colonies.
Protoecial cone flared to overlap protoecium early in astogeny, establishing circular colony with autozooids radiating from colony center. Autozooids budded only in continuous budding zone around colony perimeter, first indicated by nearly parallel, radiating interiorwall ridges on inner portion of perimetrical basal wall lamina. Ontogenetically older autozooids developing exterior wall including short to long, isolated peristomes aligned in poorly organized radiating rows. Ontogenetically oldest autozooids (in colony center) capped by single, flat, slightly submerged terminal diaphragms.
Gonozooids with broad, strongly inflated brood chambers, parallel with colony margin and brood chambers laterally encompassing several rows of autozooecial peristomes. Ooeciostome on peripheral margin of gonozooid, short, recurved, usually slightly more slender than autozooidal peristomes. Ooeciopores may be circular or laterally elongate. Multiple gonozooids possible, with brood chambers formed simultaneously, usually in single band near colony perimeter.
Interior wall surfaces apparently lacking spines but with small, uniformly spaced bumps. Pseudopores in exterior wall partially occluded by centripetally converging spines. Pseudopores more densely spaced on brood chamber surface than on autozooids.
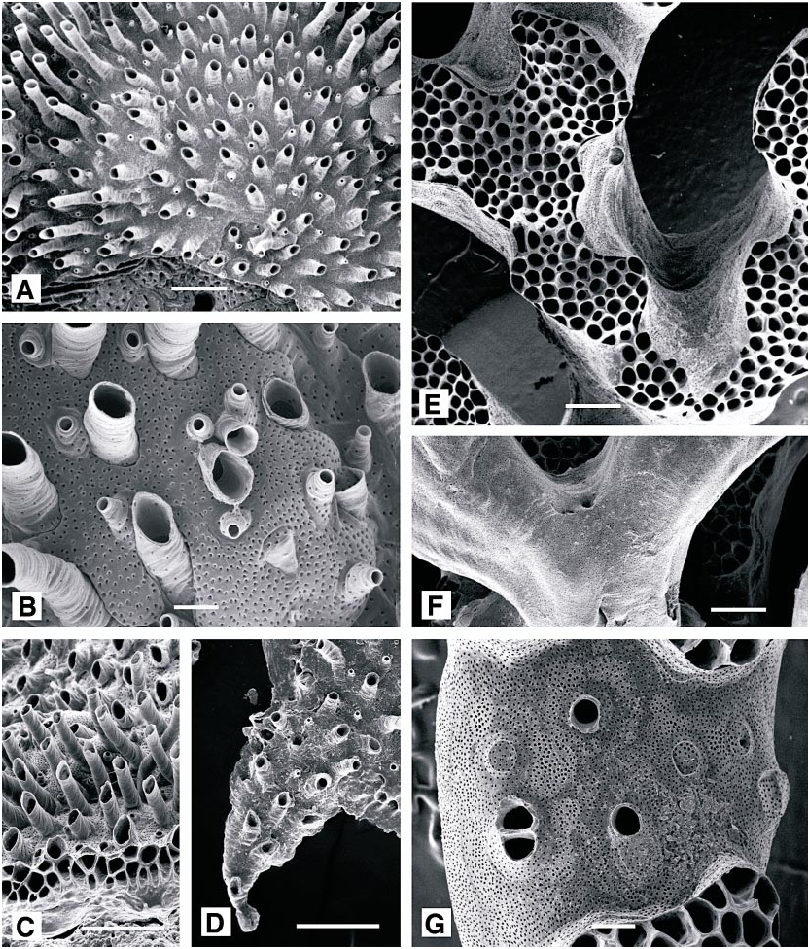
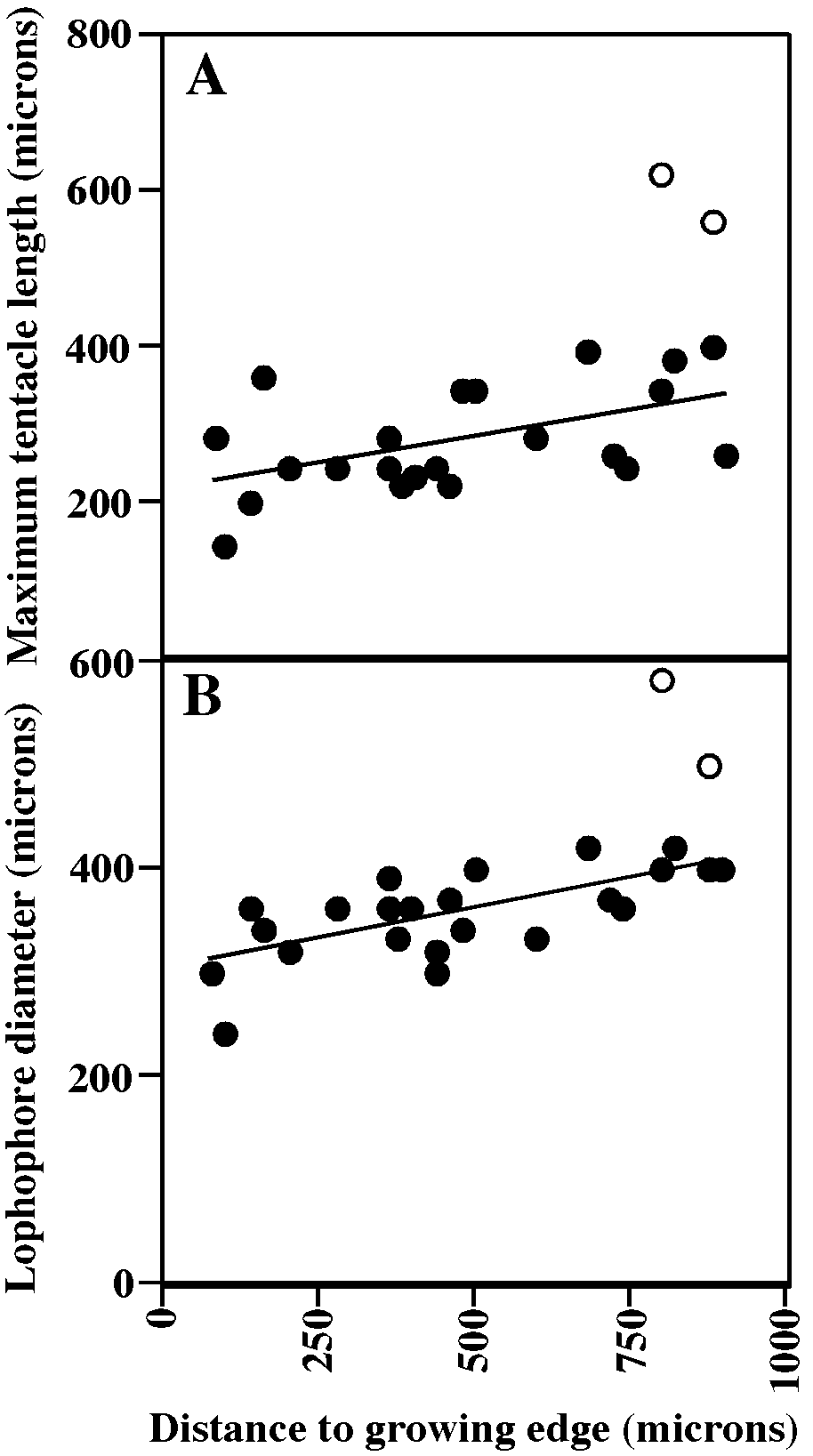
Tentacles clear, 10; tentacles short (fig. 54A) and lophophores small (fig. 54B), conical along colony margin, grading to campylonemidan and obliquely truncate in ontogenetically older autozooids; only marginal autozooids with functioning polypides. Short introverts (longest in ontogenetically oldest autozooids) visible in some colonies (fig. 54A).
REMARKS: Nielsen (1970) described embryology, settlement, and metamorphosis of P. patina , but either there is variation in ten tacle number between the Adriatic and the North Sea (Nielsen reported 8 tentacles; specimens studied here have 10), or two different species were studied by Nielsen and by us. Embryos were reported June–August for specimens from the Isle of Man ( Eggleston, 1969), and embryos were seen in our specimens during June. Large, fertile colonies commonly have abundant small colonies within 2 cm of their margins, apparently offspring that settled near the maternal colony.
OCCURRENCE: Plagioecia patina ranges between 8 m and at least 75 m depth in the Mediterranean ( Harmelin, 1976), where it is found in a range of orientations of substratum and intensities of light. In the Mediterranean and the northern Adriatic, diverse hard substrata are occupied, particularly bivalve shells (especially undersurfaces; Mc Kinney, 2000), other calcified bryozoans, rocks from pebbles to cave surfaces and rock walls, and artificial substrata. It is also known to occur on rhizomes of Posidonia ( Harmelin, 1976) . P. patina is one of the more abundant bryozoan epibionts of Cellaria spp. within Cellaria meadows offshore of Rovinj ( McKinney and Jaklin, 2000), where it is attached to the Cellaria branches only by the central part of the colony and typically radiates from the point of attachment as relatively planar disk.
DISTRIBUTION: P. patina is common in the northeastern Atlantic, from Arctic Norway southward, and in the Mediterranean Sea. It has been reported from many other parts of the world, but such reports are thought to be in error ( Hayward and Ryland, 1985a).
MEASUREMENTS (SKELETAL): AD 102 ± 11 µm, 85–130 (4, 40), AS 207 ± 42m 110– 280 (4, 40), GL 889 ± 137, 600–1100 (7, 14), GS 2511 ± 445, 1600–3100 (7, 14), OsDMN 85 ± 15, 60–120 (7, 14), OsDMX: 94 ± 17, 65–120 (7, 14). (POLYPIDE): IH 81 ± 58 µm, 0–200 (1, 12), LDMn 345 ± 45, 260–400 (1, 11), LDMx 407 ± 47, 320–500 (2, 30), MD 15 (1, 1), TLMn 289 ± 50, 200– 340 (1, 11), TLMx 355 ± 77, 210–560 (2, 33).
Plagioecia sarniensis ( Norman, 1864) View in CoL Figure 55A–E View Fig
Diastopora sarniensis Norman, 1864: 89 . Hincks, 1887: 308.
Berenicea sarniensis :? Harmer, 1915: 114. Canu and Bassler, 1928: 65.
Microecia sarniensis: Canu, 1918: 326 .
Plagioecia sarniensis View in CoL :? Canu and Bassler, 1925: 65. Harmelin, 1976: 136. Hayward and Ryland, 1985a: 100.
DESCRIPTION (AMNH 1033): Colony white, completely encrusting, including marginal lamina, multiserial; irregularly discoidal with rejuvenated areas extending as lobes. Ancestrula and early astogenetic stage obscured by multiple small intracolony overgrowths.
Autozooids budded only in continuous budding zone around colony perimeter. Ontogenetically older autozooids developing exterior wall including long, generally isolated peristomes aligned in poorly organized radiating rows, locally in short connate series. Peristomes commonly slightly tapered toward aperture. Ontogenetically oldest autozooids (in colony center) capped by single, slightly submerged terminal diaphragms perforated centrally or slightly distally by small pore at top of inverted, funnelshaped extension. Locally, rim of aperture extended as slight peak at end of small keel on distal edge.
Rare nanozooids budded against basal lamina at growing edge of colony; peristomes as short as funnelshaped extensions of autozooidal terminal diaphragms, with diameter approximately onefourth that of autozooids.
Gonozooids with broad, strongly inflated brood chambers, parallel with colony margin and brood chambers laterally encompassing several rows of autozooidal peristomes. Ooeciostome located near peripheral margin of gonozooid; short, recurved, more slender than autozooidal peristomes. Ooeciopore may be capped by terminal diaphragm forming funnel, constricting diameter to onehalf, then broadly flared along outer edge.
Pseudopores in nonperistomial exterior wall partially occluded by centripetally converging spines. Pseudopores more densely spaced on brood chamber surface than on autozooids. Pseudopores in peristomes smaller, apparently without partial occlusion by converging spines.
REMARKS: The centrally perforated terminal diaphragms atop peristomes provide egress for a single, nonciliated tentacle of a secondary nanozooid for which no function is obvious ( Silén and Harmelin, 1974). They lack testes that would indicate male polymorphs, and their position and behavior do not indicate cleaning similar to nanozooids of Diplosolen obelium . Widely scattered primary nanozooids in P. sarniensis (and in P. patina ) have been reported previously by Silén and Harmelin (1974).
DISTRIBUTION: The species is known in the eastern Atlantic from southern Britain to Angola and as far east in the Mediterranean as the Aegean Sea.
MEASUREMENTS (SKELETAL): ADMN 98 ± 7 µm, 80–110 (1, 10), ADMX 119 ± 3, 110–120 (1, 10), AS 244 ± 36, 180–300 (1, 10), GL 520 ± 28, 500–540 (1, 2), GW 1215 ± 244, 1000–1560 (1, 4), OsDMN 60–70 (1, 2), OsDMX 80 (1, 2).
GENUS EURYSTROTOS HAYWARD AND RYLAND, 1985b
Eurystrotos compacta (Norman, 1867) Figure 55F–H View Fig
Alecto compacta Norman, 1867: 204 .
Eurystrotos compacta: Hayward and Ryland, 1985a: 94, 1985b: 1075 . Zabala and Maluquer, 1988: 171.
Diastopora suborbicularis Hincks, 1880: 464 .
Berenicea suborbicularis: Marcus, 1940: 74 .
Microecia suborbicularis: Harmelin, 1976: 122 . Zabala, 1986: 644.
DESCRIPTION (AMNH 931): Colonies encrusting, multiserial, thin, up to 5.0 X 4.6 mm, with irregular outline; consisting of arcshaped, laterally coalescent subcolonies originating by continued normal growth from a small group of ancestral zooids along edge of an older subcolony in which growth has ceased along most of arcshaped perimeter. Autozooids small, budded in quincuncial series, with clearly visible adnate proximal portions and distal peristomes extending well above the general colony surface. (Peristomes of the available specimens are corroded, so length cannot be determined.) Colony surface corrugated by fine growth lines, especially visible on peristomes. Surface of colony abundantly perforated by circular pseudopores, less abundant on the peristomial skeleton but particularly dense on brood chamber surfaces.
Gonozooids abundant, with 17 fully formed brood chambers present in the larger specimen. Brood chambers located near the outer edges of subcolonies, averaging just over one zooid row distance from outer edges (250 ± 89 µm, N = 16). Brood chamber width about twice chamber length, extending as short lobes and without engulfing peristomes of adjacent autozooids. Ooeciostomes short, recurved, and are located on the distal edge of brood chambers, distinctly smaller than autozooidal peristomes; varying from circular to oval with maximum diameter transverse.
Ancestrula small, with peristomial diameter equal to diameter of ooeciostomes. A single autozooid budded from ancestrula, succeeded by a median distal autozooid and two laterally curved distolateral zooids, initiating a budding zone that widens and recurves rapidly. The lateral ends of the marginal budding zone meet and coalesce at approximately the fifth to sixth asexually budded generation, generating a continuous perimetrical budding zone, and leaving a small oval gap on either side of primary astogenetic zone of change.
REMARKS: Only two specimens of this species were found, neither of which was alive when observed in the laboratory. They have many features in common with shallowwater colonies of Eurystrotos compacta ( Harmelin, 1976; Hayward and Ryland, 1985a), differing in having smaller brood chambers than normal for the species and that differ from British specimens in being transversely elongate rather than longitudinally elongate. They possibly belong to an undescribed species of Eurystrotos .
OCCURRENCE: On glass bottle, 25–30 m deep, west slope of Banjole.
DISTRIBUTION: E. compacta is known in the eastern North Atlantic from Denmark to southern Britain, the Mediterranean Sea, and the Adriatic Sea.
MEASUREMENT (SKELETAL): ADMN 60 ± 4 µm, 55–65 (1, 10), ADMX 65 ± 7, 60–80 (1, 10), AS 213 ± 18, 180–240 (1, 10), GL 266 ± 29, 220–320 (1, 16), GW 476 ± 58, 400–560 (1, 16), OsDMN 37 ± 3, 35–40 (1, 11), OsDMX 41 ± 3, 35–45 (1, 11).
GENUS DIPLOSOLEN CANU, 1918
Diplosolen obelium ( Johnston, 1838) View in CoL Figures 56A–D View Fig , 57 View Fig
Tubulipora obelia Johnston, 1838: 269 .
Diastopora obelia: Johnston, 1847: 277 . Heller, 1867: 123. Waters, 1879: 273.
Diplopora obelia: Jullien and Calvet, 1903: 116 , 162.
Diplosolen obelia: Canu and Bassler, 1920: 745 View in CoL . Hayward and Ryland, 1985a: 102.
Diplosolen obelium: Harmelin, 1969 a: 1183, 1976: 145 View in CoL . Zabala, 1986: 641.
DESCRIPTION (AMNH 1034, 1035; CMRR
2277): Colonies white, encrusting, thin, mul
tiserial, usually adnate with peripheral basal lamina less than 1 mm wide. Small and most large colonies circular, some large colonies with lobes; typically a few millimeters in diameter, largest colonies slightly over 10 mm. Where growing on bryozoan substrata, growing edge may grow free and arch up into hemispherical blisters over zooidal orifices of underlying substratum.
Autozooids and nanozooids regularly alternating in both longitudinal and lateral rows, distributed in highly ordered quincuncial series. Autozooidal diameter small, proximal portion adnate, distal portion as free peristome with height coordinated with that of neighbors; peristomes very long where colony or local portion of colony located in depression.
Autozooidal tentacles colorless, 10, shortest at colony margins (fig. 57A); lophophores campylonemidan, grading from smallest along colony margin to largest away from colony margin (fig. 57B) within peripheral band of functioning autozooids; peristomes of autozooids in colony centers broken off and autozooids closed by terminal diaphragm.
Nanozooids regularly budded against basal lamina, in alternation with autozooids, at growing edge of colony. Peristomes shorter than, and diameter approximately onefourth that of, autozooids. Nanozooids with one thin, colorless tentacle (devoid of cilia; Borg, 1926) approximately half the length of autozooidal tentacles.
Gonozooids with nearly equidimensional, inflated brood chambers, about same size as but less inflated than blisters where colony arches over zooidal orifices where growing on Pentapora fascialis . Brood chamber incorporating base of several peristomes of adjacent autozooids, with single curved, short ooeciostome about twothirds the diameter of autozooidal peristomes and located in midregion of brood chamber.
Exterior walls about 50 µm thick, perforated by circular to elongate oval pseudopores. Brood chamber wall more densely perforated than exterior wall of other zooids; pseudopores fewer higher on peristomes.
REMARKS: The resting position of the single tentacles of the reduced nanozooid polypides is horizontal with the colony surface, ex position ( Silèn and Harmelin, 1974), interpreted as a surfacecleaning behavior.
OCCURRENCE: Colonies grow on a variety of hard substrata, especially bivalve shells and the erect bilaminate bryozoan Pentapora fascialis . On disarticulated shells, they occur more commonly on interior surfaces but are larger and more commonly fertile on exterior surfaces (McKinney, 2000).
DISTRIBUTION: Found at inner shelf depths throughout the northeastern Atlantic from the Barents Sea to northwestern Africa and throughout the Mediterranean Sea.
MEASUREMENTS (SKELETAL): PD 82 ± 8 µm, 70–92 (3, 30), AS 277 ± 41, 220–400 (3, 30), NAD 14 ± 4, 10–24 (3, 21), GL 1172 ± 22, 980–1560 (3, 6), GW 1192 ± 27, 960–1320 (3, 6), OsD 39 ± 2, 35–40 (3, 5). (POLYPIDE): IH 0 ± 0 µm (2, 29), LD 372 ± 66, 240–580 (2, 29), MD 25–30 (2, 2), TLMn 277 ± 51, 140–240 (2, 14), TLMx 296 ± 104, 140–620 (2, 28).
tending from the nanozooid orifice toward the colony center, as if oriented by the colonial feeding current, which is centripetal and thin, adjacent to the colony surface (Mc Kinney, 1992). Intermittently, the tentacle will either rotate rapidly forward and back to the normal position, or it will make a lateral, circular sweep that ends back at the normal
FAMILY FRONDIPORIDAE BUSK, 1859 View in CoL GENUS FRONDIPORA LINK, 1807 View in CoL
Frondipora verrucosa ( Lamouroux, 1821) View in CoL Figures 56E–G View Fig , 58 View Fig
Madrépore rameux, Marsigli, 1725: 105.
Krusensterna verrucosa Lamouroux, 1821: 41 .
Frondipora verrucosa: Busk, 1875: 39 View in CoL . Waters, 1879: 279. Hincks, 1887: 308. Zabala, 1986: 654. Zabala and Maluquer, 1988: 173.
? Frondipora reticulata: de Blainville, 1834: 406 .
Frondipora maderensis Johnson, 1897: 64 .
Frondipora gracilis Canu and Bassler, 1930 a: 87 View in CoL . Harmelin, 1969 a: 1187.
DESCRIPTION (AMNH 1036, 1037; CMRR 2278): Colonies erect, highly branched, with local lobes and branch ends occupied by fascicles of interiorwalled autozooids surround ed by extensive exterior wall areas interrupt ed locally by isolated or small groups of autozooids; usually canary yellow, some orangetinted yellow. Branch widths variable, generally 1–2 mm, separated by spaces approximately equal in width; fascicles preferentially oriented on individual branches, producing a poorly to well defined frontal surface. Branches may anastomose locally. Colonies increasing in width more rapidly than in height so that larger colonies up to several centimeters across but usually extending no more than 2–3 cm from substratum. Autozooids closely packed within fascicles, elongate tubular parallel with branch axis, more abundantly originating in interior regions of fascicles than along the exteriorwalled perimeter. Each fascicle a mosaic of small, newly budded autozooids and variably elongate autozooids with fully developed diameters. Some autozooid diameters very slightly larger along the perimeter of fascicles than within the centers.
Gonozooids located between fascicles, brood chambers spreading broadly across the colony surface, commonly enveloping several isolated autozooids with short peristomes projecting above the brood chamber surface. Single ooeciostome per brood chamber oval, slightly larger than autozooecial orifices, and atop a short, slightly flared tube.
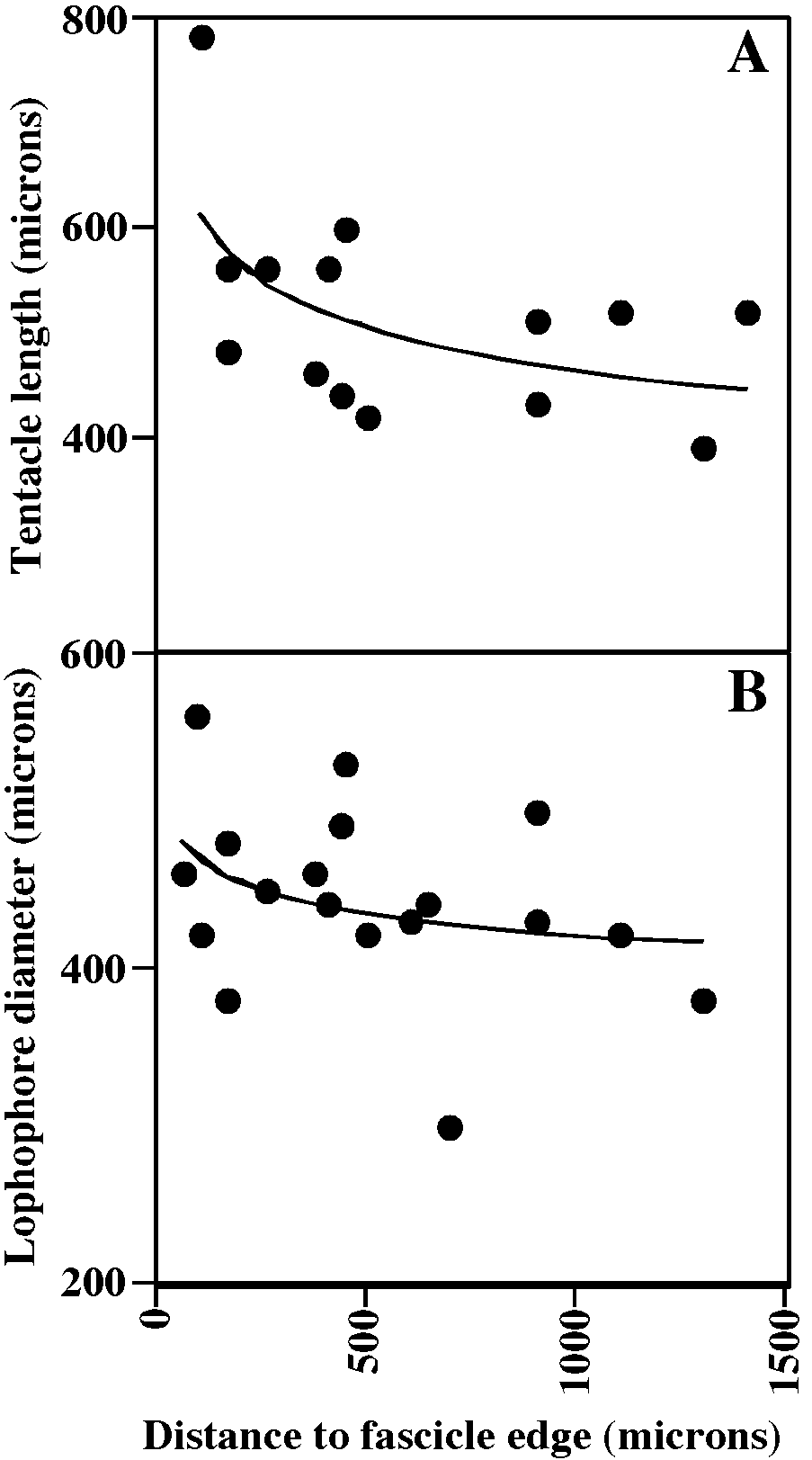
Tentacles clear or white except for irregularly distributed brown segments, 11–12, longest and constituting largest lophophores along exteriorwall margin of fascicle (fig. 58A, B); lophophores campylonemidan, most strongly obliquely truncate along exteriorwall margin of fascicle.
OCCURRENCE: In the vicinity of Rovinj, this species occurs on diverse bivalves and occasionally on other calcified bryozoans such as Cellaria and Pentapora . It was very rare during the late 1980s but had become moderately common at depths over 35 m, especially in the vicinity of the islands Pelago and Sv. Ivan, by 1997 and 1998.
DISTRIBUTION: Common in the Mediterranean, less commonly reported from the eastern North Atlantic.
MEASUREMENTS (SKELETAL): ADMN 170 ± 21 µm, 120–200 (4, 40), ADMX 194 ± 19, 160–240 (4, 40), GL 1740 ± 108, 1600– 1850 (1, 5), GW 1600 ± 162, 1450–1800 (1, 5), OsDMN 176 ± 17, 150–200 (1, 5), OsDMX 199 ± 20, 180–220 (1, 5). (POLYP IDE): IH 0 ± 0 µm (2, 32), LDMn 433 ± 73, 300–560 (2, 10), LDMx 503 ± 94, 300–660 (2, 27), MD 35 (1, 1), TLMn 387 ± 82, 240– 500 (2, 14), TLMx 596 ± 151, 390–860 (2, 20).
SUBORDER ARTICULATA BUSK, 1859 FAMILY CRISIIDAE JOHNSTON, 1847 View in CoL GENUS CRISIA LAMOUROUX, 1812 View in CoL
Crisia fistulosa Heller, 1867 View in CoL Figure 59A, B View Fig
Crisia fistulosa Heller, 1867: 118 View in CoL . Waters, 1879: 268. Friedl, 1917: 275. Harmelin, 1968: 427.
DESCRIPTION (AMNH 1038): Colonies bushy, branches robust, articulated, biserial, flat, white. Colony fragments at least 4.5 mm high, generally highly branched. Branch internodes locally giving rise to at least one lateral branch as well as distallyformed descendant internode. Internodes flat, long, straight to gently curved, composed of three to six autozooids in available material. Lateral branches almost always at third zooid. Zooids long, particularly the second in each internode, with orifices widely spaced along each side of branch. Circular autozooidal orifices alternating from sidetoside of branches, at end of short to extended peristomes only slightly curved toward frontal sides of branches.
Gonozooids absent in present material, but reported and illustrated by Harmelin (1968) as elongate cylindrical, slightly expanded proximally, with a distally placed, small ooeciopore on a short ooeciostome situated just distal to a small area lacking pseudopores.
DISTRIBUTION: First described from the Adriatic Sea, this species has been reported in relatively small numbers from the Adriatic and western Mediterranean, with a single report (Aguirrezabalaga et al., cited in Alvarez, 1994a) from the Atlantic coast of Spain.
MEASUREMENTS (SKELETAL): AD 90 ± 9 µm, 72–105 (1, 19), AS 615 ± 108, 403– 816 (1, 23), ASW 812 ± 135, 616–1210 (1, 17), BrD 274 ± 42, 206–330 (1, 8), ZL (2 nd in fascicle) 1230 ± 120, 1030–1390 (1, 9).
Crisia ramosa Harmer, 1891 View in CoL Figure 59C–F View Fig
Crisia ramosa Harmer, 1891: 134 View in CoL . Friedl, 1917: 275. Marcus, 1940: 45. Harmelin, 1968: 419. Hayward and Ryland, 1985a: 52. Alvarez, 1994a: 41.
? Crisia eburnea: Heller, 1867: 118 View in CoL . Friedl, 1917: 275.
DESCRIPTION (AMNH 1039, 1040; CMRR 2279): Colonies bushy, branches articulated, biserial, flat, white. Colonies up to 2 cm high, generally highly branched, with branch internodes commonly giving rise to one or more lateral branches as well as distallyformed descendant internode. Internodes flat, long, straight to gently curved, usually composed of 10 to 20 autozooids, except smaller number associated with rhizoids in basal segments. Lateral branches almost always at third zooid or higher, with second lateral branch, if present, about 5 zooids farther along. Circular autozooidal orifices alternating from sidetoside of branches, at end of short peristomes gently curved toward frontal sides of branches.
Gonozooids large, with distal inflated brood chambers. Ooeciopores circular, at end of frontally directed, flared ooeciostome ap proximately centered at distal end of brood chamber. Each gonozooid generally situated near middle of fertile internode, distal to first and commonly beyond second lateral branch, as previously noted by Ryland (2000). Fertile colonies collected from June to November (i.e., during all times of year when collections were made).
Adnate attachment zooids (‘‘rhizoids’’) abundant, giving rise to multiple erect branch systems. Some adnate zooids with terminal orifices giving rise to erect branch systems, while others are endtoend kenozooids, as described by Silén (1977) for Crisia cornuta .
Tentacles clear, 8; lophophores conical to flaring conical, radially symmetrical. Base of lophophore generally at orifice or within peristome, but unusually may be up to 0.04 mm above the orifice.
OCCURRENCE: The species occurs in the northern Adriatic on bryozoans (especially Cellaria and Pentapora ), shells, skeletalized and fleshy algae, Microcosmus , and firm human ejecta such as plastic and glass. C. ramosa was probably referred to by Heller (1867: 118) as Crisia eburnea (Linnaeus) , the only one of the several species of Crisia that he noted to be abundant in the Adriatic.
DISTRIBUTION: C. ramosa ranges throughout the Mediterranean and in the Atlantic from northeast England to the Azores and Cape Verde Islands.
MEASUREMENTS (SKELETAL): AD 69 ± 9 µm, 60–80 (5, 52), AS 354 ± 38, 260–420 (3, 30), ASW 412 ± 63, 300–500 (3, 30), BrD 256 ± 37, 180–340 (4, 34), GL 655 ± 59, 580–740 (3, 6), GW 507 ± 59, 460–600 (3, 6), OsD 111 ± 14, 85–120 (3, 6). (POL YPIDE): IH 1 ± 7 µm, 0–40 (4, 35), LD 349 ± 51, 240–460 (4, 34), MD 22 ± 5, 20–30 (3, 4), TL 281 ± 54, 160–420 (4, 30).
Crisia recurva Heller, 1867 View in CoL Figure 59F–J View Fig
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Tubulipora patina Lamarck, 1816: 163
| HAYWARD, PETER J. & McKINNEY, FRANK K. 2002 |
Diplosolen obelium : Harmelin, 1969 a: 1183, 1976: 145
| Zabala, M. 1986: 641 |
Eurystrotos compacta: Hayward and Ryland, 1985a: 94 , 1985b: 1075
| Zabala, M. & P. Maluquer 1988: 171 |
| Hayward, P. J. & J. S. Ryland 1985: 94 |
| Hayward, P. J. & J. S. Ryland 1985: 1075 |
Microecia suborbicularis:
| Zabala, M. 1986: 644 |
| Harmelin, J. - G. 1976: 122 |
Berenicea suborbicularis:
| Marcus, E. 1940: 74 |
Plagioecia sarniensis
| Hayward, P. J. & J. S. Ryland 1985: 100 |
| Harmelin, J. - G. 1976: 136 |
| Canu, F. & R. S. Bassler 1925: 65 |
Diplosolen obelia :
| Hayward, P. J. & J. S. Ryland 1985: 102 |
| Canu, F. & R. S. Bassler 1920: 745 |
Berenicea sarniensis
| Canu, F. & R. S. Bassler 1928: 65 |
| Harmer, S. F. 1915: 114 |
Diplopora obelia :
| Jullien, J. & L. Calvet 1903: 116 |
Frondipora maderensis
| Johnson, J. Y. 1897: 64 |
Crisia ramosa
| Alvarez, J. A. 1994: 41 |
| Hayward, P. J. & J. S. Ryland 1985: 52 |
| Marcus, E. 1940: 45 |
| Friedl, P. H. 1917: 275 |
| Harmer, S. F. 1891: 134 |
Diastopora suborbicularis
| Hincks, T. 1880: 464 |
Frondipora verrucosa: Busk, 1875: 39
| Zabala, M. & P. Maluquer 1988: 173 |
| Zabala, M. 1986: 654 |
| Hincks, T. 1887: 308 |
| Waters, A. W. 1879: 279 |
| Busk, G. 1875: 39 |
Crisia fistulosa
| Friedl, P. H. 1917: 275 |
| Waters, A. W. 1879: 268 |
| Heller, C. 1867: 118 |
Crisia eburnea :
| Friedl, P. H. 1917: 275 |
| Heller, C. 1867: 118 |
Diastopora sarniensis
| Hincks, T. 1887: 308 |
| Norman, A. M. 1864: 89 |
Diastopora obelia :
| Waters, A. W. 1879: 273 |
| Heller, C. 1867: 123 |
| Johnston, G. 1847: 277 |
Tubulipora obelia
| Johnston, G. 1838: 269 |
Frondipora reticulata :
| De Blainville, H. M. 1834: 406 |
Krusensterna verrucosa
| Lamouroux, J. V. F. 1821: 41 |
Tubulipora patina
| Marcus, E. 1940: 73 |
| Hincks, T. 1880: 458 |
| Lamarck, M. le 1816: 163 |