Lepralia fissa Busk, 1856: 308
|
publication ID |
https://doi.org/10.1206/0003-0090(2002)270<0001:NABFTV>2.0.CO;2 |
|
persistent identifier |
https://treatment.plazi.org/id/03D1878C-192B-FFC8-FF90-C5A8FE53C541 |
|
treatment provided by |
Felipe |
|
scientific name |
Lepralia fissa Busk, 1856: 308 |
| status |
|
Lepralia fissa Busk, 1856: 308 .
Schizotheca fissa Hincks, 1880: 284 View in CoL . Gautier, 1962: 223. Zabala, 1986: 543. Zabala and Ma
luquer, 1988: 150. Hayward and Ryland, 1999:
382.
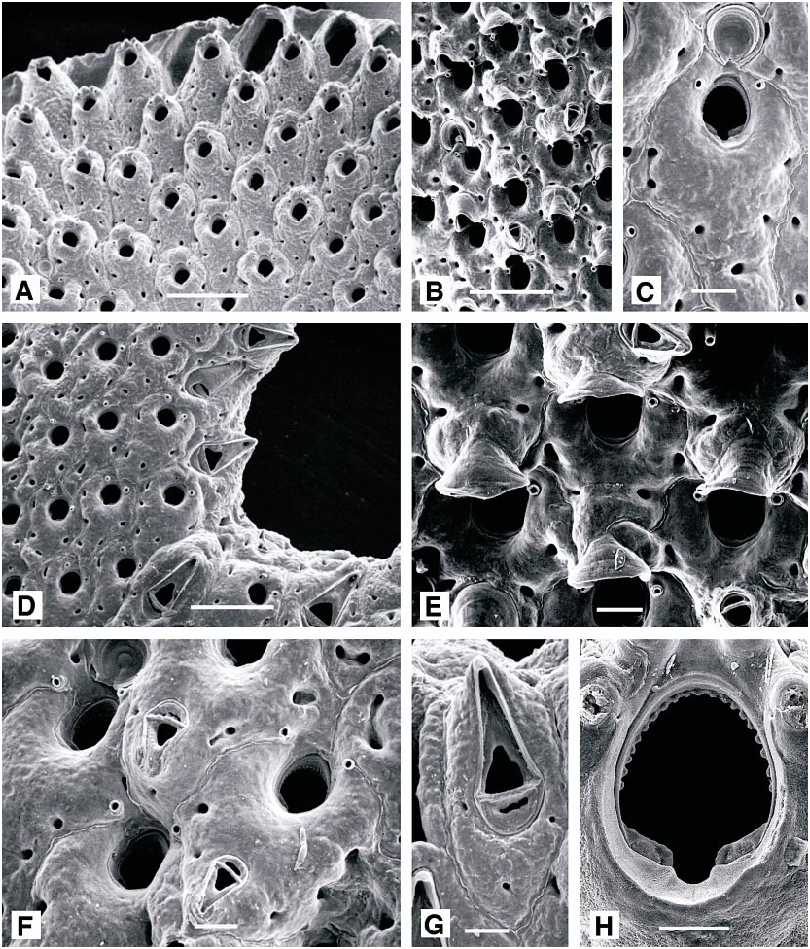
DESCRIPTION (AMNH 942, 1009, 1010; CMRR 2270): Colonies translucent, encrusting, multiserial, unilaminar, small, rounded patches. Autozooids oval to hexagonal, slightly convex, with indistinct boundaries. Frontal shield thickly calcified, finely granular, becoming coarser in later ontogeny; with few (1–3) indistinct marginal pores. Primary orifice orbicular, with denticulate distal rim and small, Ushaped proximal sinus. Peristome well developed, cylindrical, projecting well above frontal shield; six long, slender spines evenly spaced around distal and lateral borders (four in ovicellate zooids); midproximally, rim bears short, Ushaped notch, continued on inner distal face of peristome as deep groove. Avicularia vicarious, cystid about as large as an autozooid; rostrum triangular, directed distally. Ovicell elongate oval, recumbent on distally succeeding autozooid, smooth surfaced, imperforate, with elongate frontal fissure; at first prominent, later immersed in thickened calcification; some with avicularium on distal surface.
DISTRIBUTION: Often common on biogenic carbonates, S. fissa is widespread throughout the Mediterranean, in shallow detritic environments. It ranges north to the southwest British Isles, and south to the Cape Verde Islands.
MEASUREMENTS (SKELETAL): AL 376 ± 106 µm, 207–528 (3, 9), AW 225 ± 52, 171–322 (3, 9), DO 333 ± 30, 293–395 (3, 25), OL 78 ± 5, 72–86 (1, 10), OW 83 ± 7, 74–92 (1, 10), OvL 183 ± 27, 149–242 (2, 16), OvW 211 ± 18, 189–249 (2, 16), ZL 436 ± 44, 364–510 (3, 22), ZW 290 ± 20, 251– 322 (3, 22).
Schizotheca serratimargo ( Hincks, 1886) View in CoL Figures 45A–H View Fig , 46 View Fig
Schizoporella serratimargo Hincks, 1886: 268 .
Schizotheca serratimargo: Zabala and Maluquer,
1988: 148.
DESCRIPTION (AMNH 1011–1013; CMRR 2271): Colonies orange, erect, bilaminate, branching and anastomosing, developing rigid, threedimensional, reticulate structures; branches of variable width (3.6 ± 1.0 mm, N = 221), dividing frequently and irregularly. Autozooids hexagonal to irregularly polygonal, becoming more rounded in later ontogeny but remaining distinct, each bounded by thin, raised sutures. Primary orifice longer than wide; anter semielliptical, with finely denticulate inner rim; poster shallowly concave with an indistinct median sinus, emphasized and deepened by thick, prominent condyles extending on each side from proximolateral corner to edge of sinus. Two widely spaced distal oral spines present, persisting in late ontogeny. Frontal shield thickly calcified, convex, nodular with few (<6) irregularly distributed marginal pores. Adventitious avicularia sporadic, often absent over young areas of the colony while frequent on older portions; rostrum elongate triangular, distally hooked, acute to frontal plane and with variable orientation. Large vicarious avicularia present along branch margins, cystid as large as an autozooid, rostrum almost as long, elongate triangular and distally hooked; palate with a triangular foramen, crossbar thick but without a columella. Ovicell hyperstomial, longer than wide, rather small; aperture widely open, extending distally as a triangular fissure; ooecial cover obscures ovicell in later ontogeny.
Tentacles light orange, 12–15, usually 14; lophophores bellshaped, radially symmetrical with uniform tentacle lengths away from branch margins, grading to strongly obliquely truncate with increasingly unequal tentacle lengths and longer introverts along branch margins (fig. 46), which serve as elongate excurrent strips; lophophores also obliquely truncate immediately adjacent to chimneys, which develop on broadest bifoliate surfaces ( McKinney, 1989).
DISTRIBUTION: Described from the Adriatic, where it is common on hard substrata in shallow inshore habitats, S. serratimargo has also been found to be abundant on the Catalan coast ( Zabala, 1986) and is probably widely distributed throughout the Mediterranean. There is a single record of it from the western English Channel ( Hayward and Ryland, 1999).
MEASUREMENTS (SKELETAL): AAL 177 ± 37 µm, 134–252 (2, 15), AAW 103 ± 28, 73–177 (2, 15), DO 356 ± 36, 295–439 (2, 20), OL 110 ± 7, 99–125 (2, 20), OW 96 ± 5, 88–105 (2, 20), OvL 171 ± 13, 154–204 (2, 20), OvW 174 ± 10, 160–193 (2, 20), SL 11 ± 2, 7–17 (2, 20), SW 21 ± 4, 15–28 (2, 20), VAL 531 ± 58, 437–656 (2, 19), VAW 335 ± 38, 267–402 (2, 19), ZL 502 ± 55, 421–641 (2, 20), ZW 345 ± 19, 310–379 (2, 20). (POLYPIDE): IH 125 ± 63 µm, 30–240 (6, 52), LDMn 560 ± 107, 420–800 (4, 37), LDMx 638 ± 143, 380–940 (6, 62), TLMn 472 ± 88, 360–640 (6, 42), TLMx 689 ± 186, 400–1060 (6, 53).
CLASS STENOLAEMATA BORG, 1926 View in CoL
ORDER CYCLOSTOMATA BUSK, 1852
SUBORDER TUBULIPORINA MILNE EDWARDS, 1838 View in CoL
FAMILY TUBULIPORIDAE JOHNSTON, 1838 View in CoL
GENUS TUBULIPORA LAMARCK, 1816 View in CoL
Tubulipora liliacea ( Pallas, 1766) View in CoL Figures 47A–D View Fig , 48 View Fig
Millepora liliacea Pallas, 1766: 248 .
Tubulipora liliacea: Harmer, 1898: 90 View in CoL . Harmelin, 1976: 171. Hayward and Ryland,1985a: 74. Zabala and Maluquer, 1988: 176.
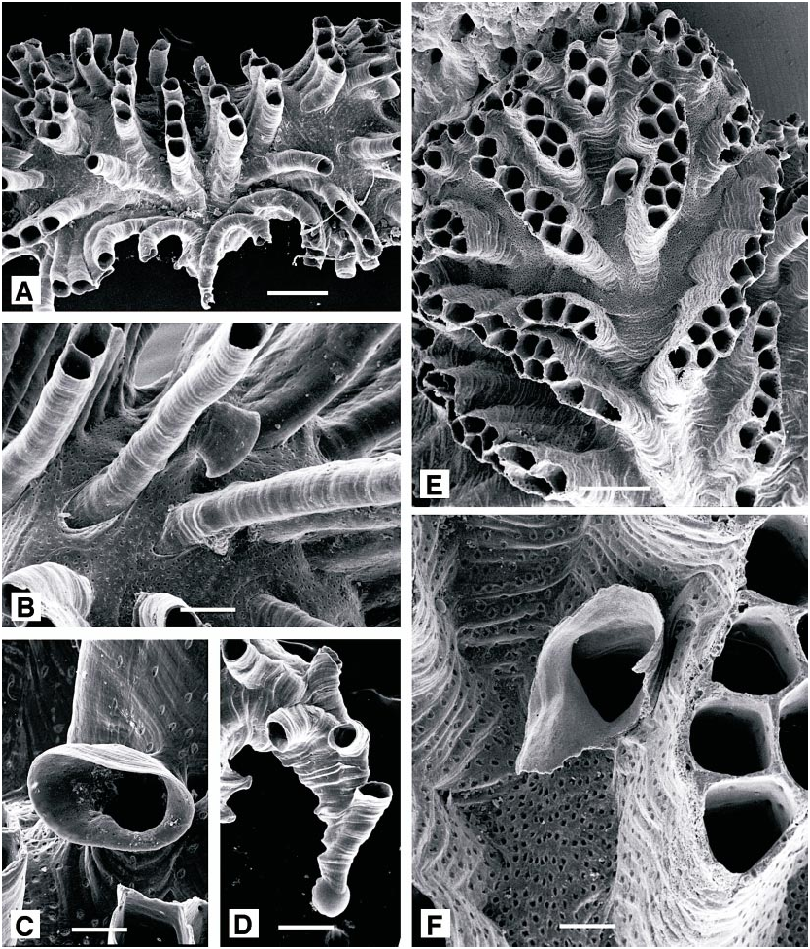
DESCRIPTION (AMNH 1014–1016; CMRR 2272): Colonies encrusting to semierect, commonly extending as two multiserial lobes diverging laterally from axis of ancestrula, up to at least 8 mm in the vicinity of Rovinj; usually violetcolored. Autozooids with long peristomes, longest in the most cryptic microenvironments. Peristomes isolated in early astogenetic portion of colony, then organized into singlerow fascicles radiating from centers of lobes. Peristomes contiguous and appressed within fascicles but in some, independent at distal ends. Individual fascicles constituted by 2 to 8 peristomes in present material and elsewhere in the Mediterranean ( Harmelin, 1976), but up to 12 in British specimens ( Hayward and Ryland, 1985a). Spacing between fascicles variable. Autozooidal apertures oval where isolated and elongate polygonal where connate.
Gonozooids distinctly inflated, centered in lobes, more densely perforated and with larg er pseudopores than autozooids. More than one ooeciostome present in some lobes, suggesting multiple confluent or contiguous gonozooids. Ooeciostomes located distally, adjacent to base of second or third peristome, usually located against side of fascicle facing distal end of lobe, curved, opening laterally or downward, toward surface of gonozooid; ooeciostomes constricted at some point to about the diameter of autozooids, then terminating in a broadly flaring oval hood.
Protoecium hemispherical, about 120 µm diameter, giving rise to laterally oriented, elongate tubular portion of ancestrula. Protoecial cone gradually widening, generally curved right or left and consisting of about four generations of autozooids with isolated peristomes. At distal end of protoecial cone, budding rate increased rapidly, particularly along lateral margins (establishing two laterally diverging lobes), and transversely oriented fascicles of peristomes appearing abruptly, establishing astogenetic zone of repetition.
Tentacles clear but locally colored by purple or brown granules, 11; lophophores small, conical, radially symmetrical along growing margin to campylonemidan, progressively larger and more strongly obliquely truncate due to increasingly unequal tentacle lengths toward chimneys in colony centers (fig. 48A), although tentacles are arrayed to maintain roughly circular shapes (fig. 48B).
OCCURRENCE: The species is widely distributed, occurring on bivalve shells, bryozoans, rocks, and Microcosmus . In the present material, colonies on bivalve shells, rocks, and Pentapora fascialis usually are adnate, while those on Cellaria and Microcosmus usually are semierect. Specimens that occur on bivalve shells are overwhelmingly located on the shell interiors, indicating a strong preference for cryptic microenvironments ( Harmelin, 1976; McKinney, 2000).
DISTRIBUTION: T. liliacea is widespread in the eastern North Atlantic and Mediterranean; it has also been reported from the Barents Sea ( Kluge, 1962, 1975) and from the northeastern coast of North America ( Osburn, 1912), though these more distant records need to be confirmed. Harmelin (1976) recorded the species at depths up to 60 m in the western Mediterranean, and found that variations in the robustness of colonies and orientation of the ooeciostome were related to the physical environment.
MEASUREMENTS (SKELETAL): ADMN 142 ± 17 µm, 120–180 (4, 40), ADMX 169 ± 19, 120–200 (3, 30), Gap 327 ± 35, 240–420 (4, 31), OsDMN 213 ± 23, 200–240 (2, 3), OsDMX 328 ± 32, 270–360 (3, 6). (POLYP IDE): IH 0 ± 0 µm (6, 67), LMN 503 ± 144, 260–880 (6, 41), LMX 520 ± 133, 320–920 (6, 43), MD 25 ± 0 (2, 2), TLMn 462 ± 121, 160–720 (6, 54), TLMx 601 ± 204, 300–1080 (6, 59).
Tubulipora plumosa Harmer, 1898 View in CoL Figure 47E, F View Fig
Tubulipora plumosa Harmer, 1898: 105 View in CoL . Harmelin, 1976: 177. Hayward and Ryland, 1985a: 80. Zabala, 1986: 672. Zabala and Maluquer, 1988: 176.
Tubulipora flabellaris Johnston, 1847: 274 View in CoL . Busk, 1875: 25 (pars, fide Harmer).
Tubulipora fimbria: Hincks, 1880: 448 .
DESCRIPTION (AMNH 1017): Colonies encrusting, white, extending as one or more short, multiserial lobes. Autozooids in oneto tworow fascicles; up to at least 18 completely connate autozooids per fascicle. Autozooids relatively large, elongate polygonal to semipolygonal in cross section. Autozooidal fascicles diverging to right and left from axis of lobe. Fascicles highest at inner ends, where composed of oldest autozooids, gradually diminishing to zero height at outer ends, in circumcolony budding zone.
Gonozooids located distally within lobes, their brood chambers ramifying between several adjacent fascicles. Ooeciopore located at distal end of a centrally located ramification. Ooeciopores adjacent to second or third peristome within fascicle, with broadly flared, highly elongate oval hoods oriented away from surface of brood chamber. Colony surfaces, including fascicles, finely but clearly corrugated, with abundant circular to distally tapered triangular pseudopores. Surfaces of brood chambers poorly corrugated, with very abundant pseudopores. Distance between pseudopores on roofs of brood chambers approximately equaling pseudopore diameter.
Tentacles clear, 11; lophophores bellshaped, radially symmetrical to obliquely truncate and campylonemidan, grading from small along colony margin to largest and most strongly obliquely truncate bordering chimneys in colony centers.
REMARKS: Authorship of this species is commonly attributed to Thompson in Harm er (1898), because Harmer adopted the trivial name plumosa that was used by Thompson for specimens from Ireland sent to Johnston (cited in Johnston, 1847). However, Harmer (1898) based his description on specimens from the coast of Devon, leading Ryland (1963: 7) to designate one of Harmer’s specimens from Devon, housed in the Museum of Zoology, Cambridge, as lectotype.
DISTRIBUTION: T. plumosa is known from the eastern North Atlantic, from Norway to the Mediterranean.
MEASUREMENTS (SKELETAL): ADMN 181 ± 20 µm, 160–220 (1, 10), ADMX 194 ± 21, 160–220 (1, 10), Gap 420 ± 85, 230–530 (1, 10), OsDMN 230 (1), OsDMX: 440 (1). (POLYPIDE): IH 0 ± 0 µm (1, 10), LD 424 ± 82, 320–556 (1, 10), TLMn 358 ± 112, 200– 540 (1, 10), TLMx 402 ± 112, 200–600 (1, 11).
GENUS EXIDMONEA DAVID, MONGEREAU, AND POUYET, 1972
Mongereau (1969) introduced the name Exidmonea for erect idmoneid species that
differ from the Middle Jurassic type species of Idmonea Lamouroux 1821 , which is predominantly encrusting and has elliptical branch cross sections in the occasional erect portions. Additionally, species of Exidmonea lack proximally directed kenozooids on the flat abfrontal surface of the branches and therefore differ from Idmidronea Canu and Bassler, 1920 , which has them. As noted by Taylor and Voigt (1992) Mongereau did not choose a type species from among the species that he listed for Exidmonea in 1969, so the first valid use of Exidmonea was by David et al. (1972), when the type species Exidmonea atlantica AUCT. was first specified.
Exidmonea triforis ( Heller, 1867) View in CoL Figures 49A–H View Fig , 50 View Fig
Idmonea triforis Heller, 1867: 120 . Waters, 1879:
271. Waters, 1922: 12. Friedl, 1917: 276.
Idmonea meneghinii Heller, 1867: 120 .
Idmonea concav a: Waters, 1879: 271.
Idmonea marionensis: Waters, 1879: 270 .
Tubulipora atlantica: Harmer, 1915: 124 . Cook,
1968: 230.
Idmidronea atlantica: Osburn, 1947: 5 View in CoL . Harmelin,
1976: 182. Zabala, 1986: 658.
Exidmonea atlantica: David et al., 1972: 84 View in CoL . Mc
Kinney, 1991a: 264. McKinney, 1991b: 437.
LECTOTYPE (chosen here): UIIZ 321.
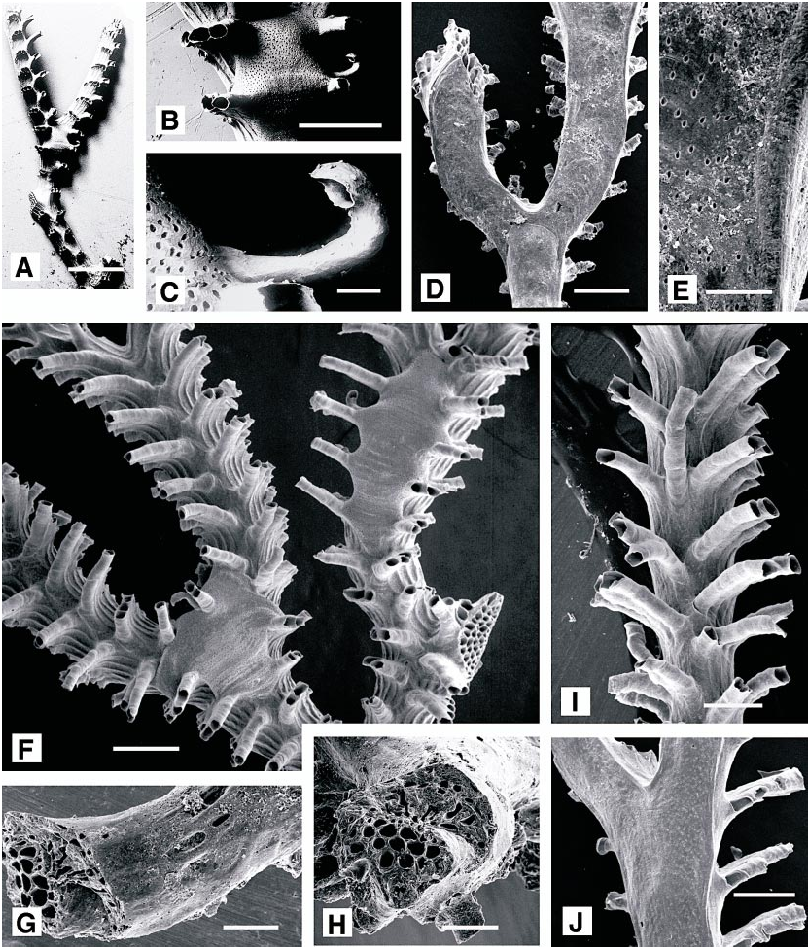
DESCRIPTION (AMNH 1018, 1019; CMRR 2273): Delicate erect planar or nearly planar fanshaped, white colonies of dichotomous branches. Branches subtriangular in cross section, with flat abfrontal surface along which the zooids originated and keeled frontal surface. Autozooids divergent at low angle from abfrontal surface toward frontal surface; those emergent near branch midline being longest. Autozooids in singlerow connate fascicles, each fascicle extending across half of frontal surface, from central keel to lateral edge. Typically four, less commonly three or five, autozooids within a row. Fascicles typically curved proximally near lateral branch margin. Peristomes curved, bending away from the branch midline. Peristomes near branch midline longer, usually extending laterally beyond those closer to lateral branch margin.
Gonozooids centered on frontal branch surfaces, most commonly situated immediately proximal to branch bifurcations Brood chambers engulfing lower portions of peristomes in two to four fascicles on each side of branch; ooeciopore situated at the end of short, approximately 180°curved ooeciostome with smaller diameter than peristomes of typical autozooids. Ooeciopores typically opening downward, toward branch surface. Ooeciostomes typically located within distal portion of brood chamber, against distal side of a fascicle of autozooidal peristomes.
Tentacles clear, 10; lophophores of zooids located along branch keel highly asymmetrical and campylonemidian, with longest tentacles on the side closest to the branch center. Size of lophophores and constituent tentacles decreased, degree of asymmetry progressively decreased, and flare of the tentacles progressively increased, in autozooids closer to lateral branch margin (fig. 50). Due to the shapes, sizes, placement, and orientation of lophophores, those from branch center extending laterally farther than those at branch margins, with their highly obliquely truncate outer end oriented toward abfrontal side. They therefore generate a unified colonial feeding current that draws nutrientbearing water from the abfrontal side of branches and ejects filtered water along the frontal surface ( McKinney, 1991a, 1991b).
REMARKS: We designate as lectotype Heller’s specimen illustrated here as fig. 49A–C (UIIZ 321). This species has long been referred to as Idmonea atlantica Forbes in Johnston, 1847, or as I. atlantica auctt. When David et al. (1972: 84) specified Exidmonea atlantica AUCT. as type species of Exidmonea , they established a new species, separate from Idmonea atlantica Forbes in Johnston, 1847. Although David et al. (1972) listed Miocene (Burdigalian) specimens as the material in hand when they established the new species, they did not designate a holotype. However, type material exists for two of Heller’s (1867) species that have precedence for at least the Recent Idmonea atlantica auctt. of the Adriatic and probably beyond (Mediterranean, Atlantic). Specimens of Exidmonea from the vicinity of Rovinj encompass the attributes present in Heller’s specimens of Idmonea meneghinii (UIIZ 320) and Idmonea triforis . We prefer to use the name triforis for the species, since most of our specimens are gracile rather than robust.
The lectotype of E. triforis has proximally directed kenozooids on the proximal end of its reverse surface. The proximal end of the specimen is slightly expanded and apparently is broken from just above its base of attachment, which is not preserved. In other specimens, the base of attachment is composed largely of kenozooids that lap a short distance up around the base of the erect portion. Beyond the short distance of kenozooidal thickening of the proximal end, the reverse side has no kenozooids.
Robustness of colonies is strongly influenced by environment and microenvironment. In a study of growth habits of E. triforis across several environments, from obscure parts of caves to relatively exposed shallow terrigenous and rocky substrata, Harmelin (1973b) found length between bifurcations to be highly variable, and bifurcation angle, width of ooeciostome, width of branches, and number of zooecia per fascicle to be moderately variable. Several of these variable branch characteristics interact to produce more irregular, delicate colonies in environments such as caves and more geometrically regular, though often smaller, colonies with more densely spaced, thicker branches in the more exposed environments. Size of gonozooids, and especially size of apertures and spacing between adjacent rows of apertures, were stable across environments.
DISTRIBUTION: E. triforis is widespread on rock and skeletal substrata in the Mediterranean Sea and cold temperate Atlantic Ocean.
MEASUREMENTS (SKELETAL): AD 83 ± 8 µm, 70–100 (3, 30), PD 105 ± 9, 90–120 (3, 30), ASW 201 ± 24, 160–240 (3, 30), RS 405 ± 48, 300–500 (3, 30), GL 1408 ± 284, 1020–2120 (3, 12), GW 793 ± 253, 480–1340 (3, 12), OsD 76 ± 13, 60–100 (2, 7), BW 537 ± 126, 360–800 (3, 20). (POL YPIDE): IH 0 ± 0 µm (7, 70), MD 19 ± 2.5, 15–20 (2, 4), LDMn 309 ± 80, 200–420 (4, 22), LDMx 339 ± 63, 200–460 (7, 80), TLMn 203 ± 43, 95–320 (7. 64), TLMx 308 ± 96, 90–560 (7, 88).
Exidmonea coerula ( Harmelin, 1976) View in CoL Figure 49I, J View Fig
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Lepralia fissa Busk, 1856: 308
| HAYWARD, PETER J. & McKINNEY, FRANK K. 2002 |
Exidmonea atlantica :
| David, L. & N. Mongereau & S. Pouyet 1972: 84 |
Idmidronea atlantica :
| Osburn, R. C. 1947: 5 |
Tubulipora atlantica :
| Harmer, S. F. 1915: 124 |
Tubulipora liliacea: Harmer, 1898: 90
| Zabala, M. & P. Maluquer 1988: 176 |
| Hayward, P. J. & J. S. Ryland 1985: 74 |
| Harmelin, J. - G. 1976: 171 |
| Harmer, S. F. 1898: 90 |
Tubulipora plumosa
| Zabala, M. & P. Maluquer 1988: 176 |
| Zabala, M. 1986: 672 |
| Hayward, P. J. & J. S. Ryland 1985: 80 |
| Harmelin, J. - G. 1976: 177 |
| Harmer, S. F. 1898: 105 |
Schizoporella serratimargo
| Hincks, T. 1886: 268 |
Schizotheca fissa
| Zabala, M. 1986: 543 |
| Gautier, Y. V. 1962: 223 |
| Hincks, T. 1880: 284 |
Tubulipora fimbria:
| Hincks, T. 1880: 448 |
Idmonea concav
| Waters, A. W. 1879: 271 |
Idmonea marionensis :
| Waters, A. W. 1879: 270 |
Idmonea triforis
| Heller, C. 1867: 120 |
Idmonea meneghinii
| Heller, C. 1867: 120 |
Lepralia fissa
| Busk, G. 1856: 308 |
Tubulipora flabellaris
| Busk, G. 1875: 25 |
| Johnston, G. 1847: 274 |
Millepora liliacea
| Pallas, P. S. 1766: 248 |