Simulium (Meilloniellum) adersi (Pomeroy) 1922
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3641.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:5FAD6A33-0893-434D-B4CB-A3FA89AAE82B |
|
DOI |
https://doi.org/10.5281/zenodo.6146953 |
|
persistent identifier |
https://treatment.plazi.org/id/03D28217-F446-FFDE-93BC-097A842CBB21 |
|
treatment provided by |
Plazi |
|
scientific name |
Simulium (Meilloniellum) adersi (Pomeroy) 1922 |
| status |
|
Simulium (Meilloniellum) adersi (Pomeroy) 1922 View in CoL
Simulium hirsutum var adersi Pomeroy 1922: 459 . Original description. Simulium hirsutum var adersi , de Meillon 1930: 197.
Simulium adersi, Gibbins and Loewenthal 1933: 493 . Referred to at species rank. Simulium adersi, Gibbins 1934: 57 . Detailed description and formal designation. Simulium adersi, Bequaert 1939: 120 .
Simulium adersi, Freeman and de Meillon 1953: 110.
Meilloniellum adersi, Rubtsov 1962: 1 ,496. Transferred to new genus. Simulium (Meilloniellum) adersi, Crosskey 1969: 74 . Relegated to subgenus. Simulium (Meilloniellum) adersi, Adler and Crosskey, 2013: 51 .
Diagnosis
Adults. Female mandible toothed only on inner side; pleural membrane haired; hind basitarsus with row of stouter setae; calcipala and pedisulcus present; tarsal claw with basal tooth; female abdominal tergites III–V small, rounded; male gonostyli abruptly tapered distally, ventral plate deeply emarginated posteromedially. Pupa: thoracic cuticle with small even granules, gill with three basal branches, bifurcating to 11 finely tapered filaments; abdominal sternite IX lacks grapnel hooks. Cocoon: silk threads coarse, open weave, slipper shaped, low profile, internal pocket. Larva: body pale yellowish grey; head markedly bicoloured, head spot pigmentation negative, figure 8-shaped pattern on cephalic apotome; body cuticle with fine small trichoid setae; posteroventral tubercles poorly developed, rounded; rectal scales present; anal sclerite arms short; accessory sclerites small and distinct.
Description
Adult Female.
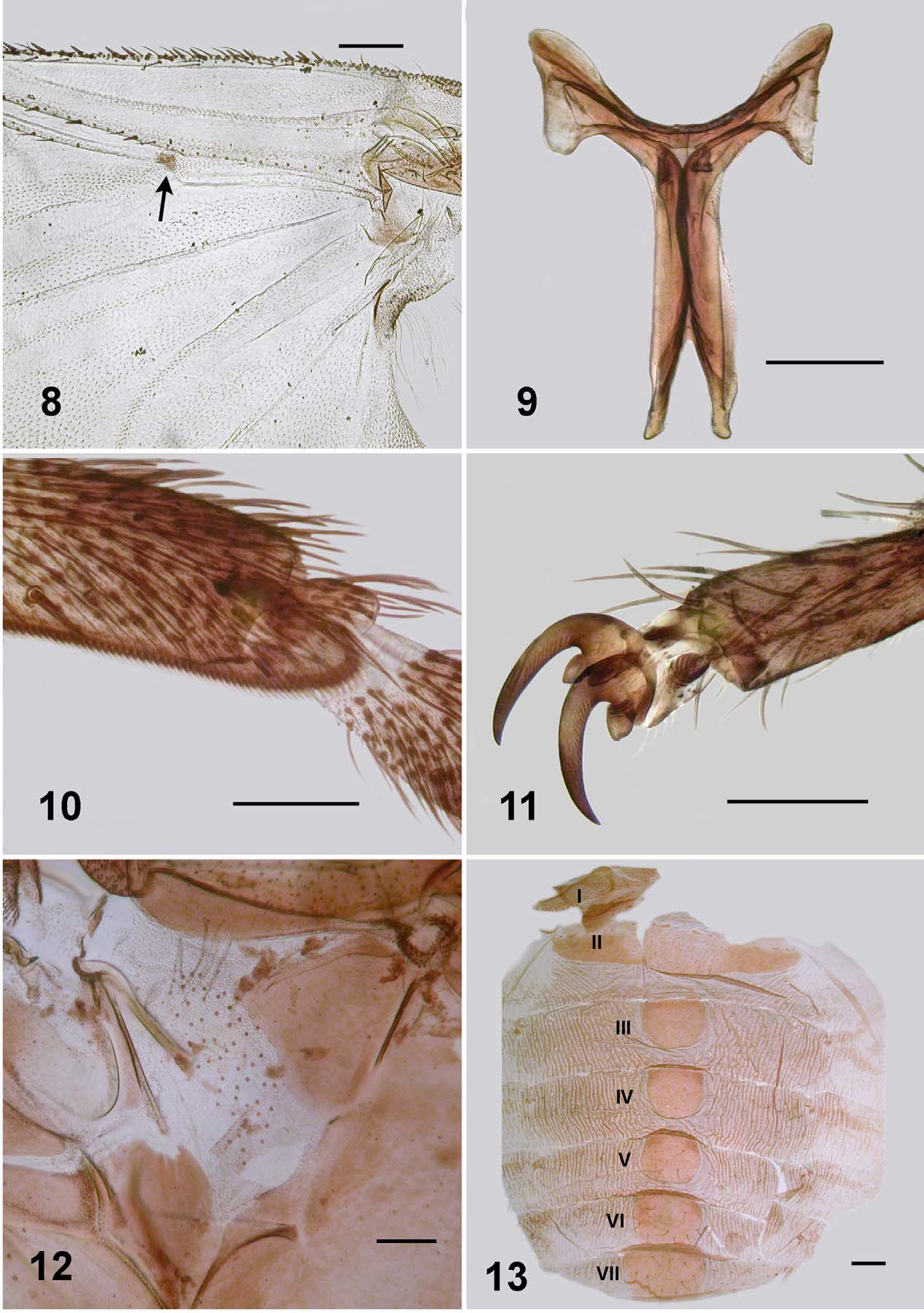
Body: general body colour in alcohol very dark reddish brown/black; total length 3.3–3.5 mm. Head ( Fig. 2 View FIGURES 2 – 7 ): width 0.60–0.64 mm; depth 0.47–0.50 mm; frons-head ratio (narrowest width of frons: greatest width of head) 1.0:6.0. Eye: interocular distance 0.09–0.11 mm; ommatidia 0.014 mm in diameter; ca. 30 rows up and across at mid-eye. Clypeus: 0.17–0.19 mm wide; dark brown; vestiture of sparse silvery scales. Antenna: length 0.46 mm; not markedly tapered, all nine flagellomeres light brown, subequal in size, wider than long, basal flagellomere larger; scape and pedicel paler. Mouthparts: poorly developed, 0.4 length of head depth; cibarium ( Fig. 5 View FIGURES 2 – 7 ) broad shallow medial space, armature absent, cornuae short, substantial, rounded; mandible ( Fig. 4 View FIGURES 2 – 7 ) narrow and elongate, lacking outer teeth, 16 inner teeth; maxillary palpus ( Fig. 6 View FIGURES 2 – 7 ), total length 0.44 mm, third article dark brown, remainder lighter, proportional length of 3rd, 4th and 5th articles 1.0:1.1:2.5; 5th article markedly elongated; sensory vesicle spherical, 0.5 times width of 3rd article, opening 0.25 times width of vesicle, lacinia with 6 inner teeth and 11 outer teeth. Thorax: length 0.95–1.2 mm; width 0.71 mm; in alcohol, postpronotal lobes concolourous with scutum, sparse pale hairs; scutum evenly dark shiny brown, vestiture of recumbent flattened silvery hairs (usually rubbed bare), presutellar depression not markedly developed, no vittae visible in alcohol; scutellum slightly lighter than scutum, vestiture of sparse long pale hairs laterally; postnotum concolourous with scutum; pleuron and plural membrane concolourous with scutum, with hairs present, but usually rubbed bare, sockets remain—see male ( Fig. 12 View FIGURES 8 – 13 ). Wing ( Fig. 8 View FIGURES 8 – 13 ): length 1.9–2.1 mm; width 0.9 mm; leading veins poorly pigmented, others virtually not; costa with spines and hairs; radius base haired, and spines more distally; radial sector basally with hairs; small area surrounding campaniform sensilla at junction of radius and median veins distinctly pigmented; vein CuA2 markedly sinuous. Haltere: white. Furcasternum ( Fig. 9 View FIGURES 8 – 13 ): anterior lateral arms flared. Legs: bicolourous with darker brown bases to femoral, tibial, and tarsal segments, yellowish-brown elsewhere; fore basitarsus about 5 times as long as its greatest breadth; hind basitarsus about 6 times as long as greatest breadth; hind basitarsus with ventral series of stout spines ( Fig. 10 View FIGURES 8 – 13 ); calcipala not markedly developed, half width of hind basitarsus; pedisulcus not markedly indented; tarsal claw elegantly curved, basal tooth moderately developed, cone-shaped, both slightly serrated ( Fig. 11 View FIGURES 8 – 13 ). Abdomen ( Fig. 13 View FIGURES 8 – 13 ): abdominal scale poorly developed with sparse, fine pale hairs, barely extended over 2nd abdominal segment; overall, pale anteriorly and evenly mottled grey elsewhere; cuticle markedly corrugated; tergites not obvious in whole animal; pleurites and sternites not expressed; tergite II broad, 5.6 times wider than long, tergite III–V as long as wide, rounded; tergites VI andVII slightly larger and also rounded; tergite vestiture of very fine sparse hairs, longer hairs on posterior tergites, not dense, but possibly heavily worn. Genitalia ( Fig. 14, 16, 17 View FIGURES 14 – 20 ): sternite VIII pigmented across, lighter medially, vestiture of longer hairs posterolaterally, medially with triads and arrays of microtrichia; hypogynial valves poorly pigmented, broadly rounded apically, vestiture of microtrichia triads; median depression broad and shallow, smoothly divergent, valves broadly rounded posteroapically; genital fork finely constructed, lateral plate elongated, apodeme not obvious (perhaps is pigmented anterior ledge of the plate), median extension of plate sharply pointed; anal lobe in lateral view bare of microtrichia, but with a few hairs, cercus broadly rounded apically. Spermatheca ( Fig. 15 View FIGURES 14 – 20 ): slightly ovoid; surface pattern absent as are internal hairs; clear area at junction of duct small. Eggs: one female, ca. 160 eggs, 0.15 x 0.09 mm in size, subtriangular in shape.
Adult Male.
Body: general colour dark blackish brown; total length 2.5–2.7 mm. Head ( Fig. 3 View FIGURES 2 – 7 ): width 0.90 mm; depth 0.70 mm. Eyes: upper larger ommatidia orange, 0.031 mm in diameter, ca. 16 across and 18 down; lower ommatidia dark brown, 0.014 mm in diameter, ca. 30 across and 35 down. Clypeus: markedly small, dark brown, pollinose in some lighting; 0.20 times as wide as head; no vestiture obvious. Antenna: total length 0.58 mm; not tapered, evenly medium brown. Mouthparts: poorly developed; length 0.33 times head depth; mandibles insubstantial, broadly tapered with apical hairs; laciniae, finely tapered apically; maxillary palpus ( Fig. 7 View FIGURES 2 – 7 ) medium brown basally, pale distally, elongate distally, sensory vesicle markedly small; 0.65 mm long, proportional lengths of 3rd, 4th and fifth articles 1.0:1.4:2.8. Thorax: length 0.9–1.2 mm; width 0.7–0.8 mm; scutum evenly dark brown/black, vestiture of sparse recumbent silvery flat hairs—normally rubbed bare; scutellum translucent and pale with sparse, long pale hairs; postscutellum concolourous with scutum; pleural membrane, haired (usually rubbed bare), but sockets remain visible (e.g., Fig. 12 View FIGURES 8 – 13 ). Wing: 2.1–2.4 mm in length, 0.9–1.2 mm at maximum width. Haltere: white. Legs: in mature specimens evenly medium brown, for immature specimens bicolorous yellowish-brown, with darker brown femoral, tibial, and tarsal bases; femur and tibia expanded slightly; hind basitarsus 4.5 times as long as greatest breadth; with row of stout spines; tarsal claw partially covered by grappling pad of 24–26 teeth. Abdomen: overall dark blackish brown; tergites occupy whole width of dorsum, more pigmented areas laterally; small pleurites and sternites posteriorly; vestiture of sparse pale hairs. Genitalia ( Fig. 18, 19, 20 View FIGURES 14 – 20 ): gonocoxa curved and smoothly tapered posteriorly, 1.3 times longer than basal width, extensive medial crenulated region posterior of base, dark brown posteriorly, pale medially, vestiture of sparse, coarse black hairs medially; gonostylus approximately 2.8 times longer than basal width, abruptly decreased in size 2/3 from base apically with single spine; ventral plate broad with major medial depression apically, moderately haired, proximal arms short, substantial; small central keel; median sclerite well expressed, narrow, slightly T-shaped posteriorly; parameres of a thin curved rod basally, thickening to terminate in substantial tooth; aedeagal membrane bare.
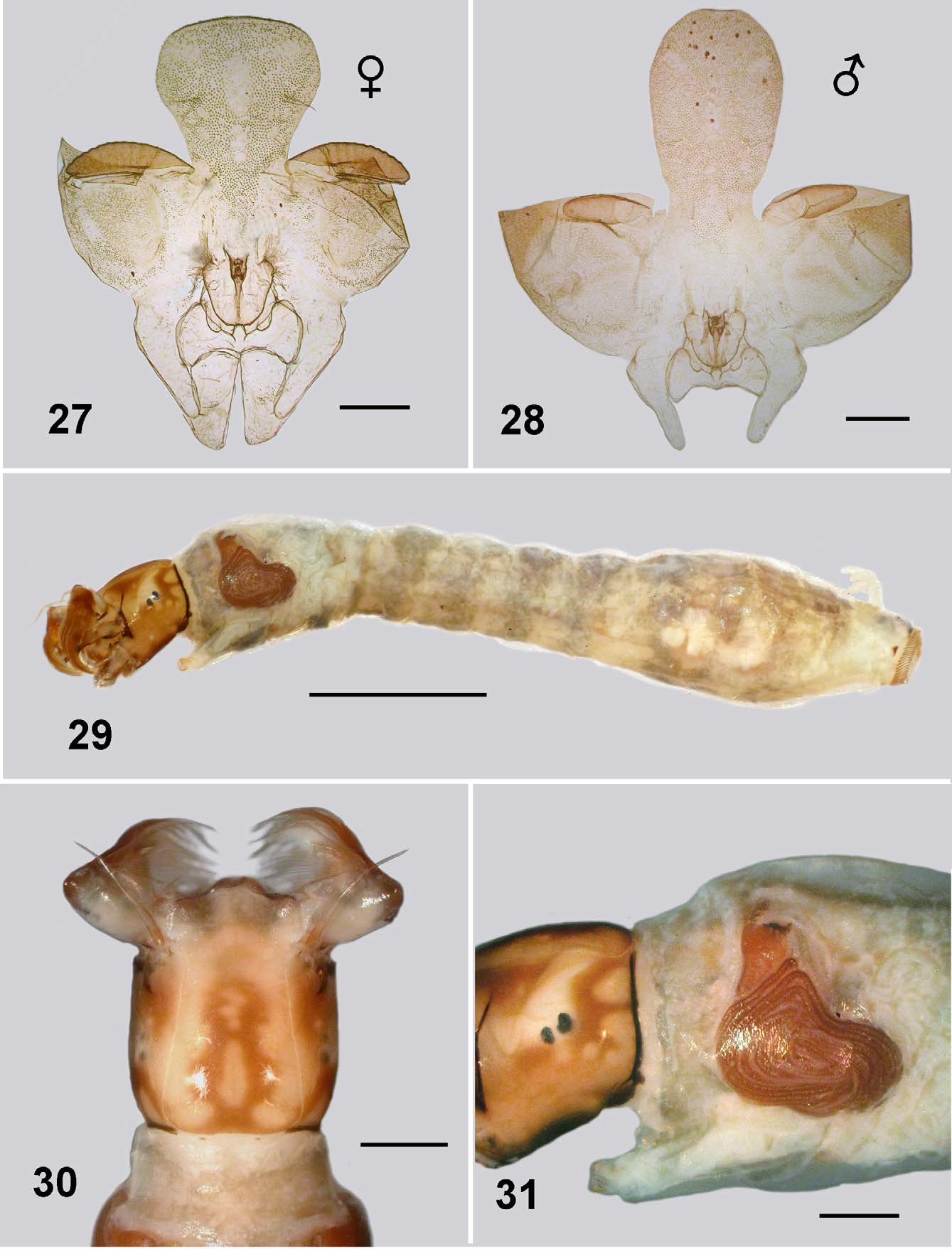
Pupa: Body length; male 2.8–2.9 mm, female 2.2–2.5 mm. Head: evenly tuberculate, including the ocular region; facial and frontal setae present, the former double, epicranial setae apparently absent; cephalic apotome of female short and broad, more tuberculate medioventrally, ratio of basal width to length 1:1.5 ( Fig. 27 View FIGURES 27 – 31 ); apotome of male ovoid, ratio of basal width to length 1:2.5 ( Fig. 28 View FIGURES 27 – 31 ); muscle scars not tuberculate. Thorax ( Fig. 24 View FIGURES 21 – 25 ). mesonotum finely tuberculate; no distinct pattern; dorsocentral setae simple, elongate. Gill ( Fig. 21, 23 View FIGURES 21 – 25 ): maximum length 2.0– 2.3 mm; basal trunk short, three branches rapidly bifurcated to produce 11 smoothly tapered filaments; ventral filaments up to half length shorter than dorsal filaments; filament surface pseudoannulated ( Fig. 25 View FIGURES 21 – 25 ). Abdomen ( Fig. 26 View FIGURE 26 ): cuticle pale and membranous; spine combs absent; posterior setae on tergite II trichoid, sparse microtubercles anteriorly; setae on tergite III and IV short curved and spine-like, microsculpture absent; setae on tergite V trichoid; tergites VI–VIII with anterior small spines directed posteriorly, lateral microsculpture of scales; sternite III with trichoid setae, sternite IV with one bifurcated spine-like seta; sternite V with two; sternites VI and VII with one bifurcated seta and one single; grapnel hooks on sternite IX absent; terminal spines short and blunt.
Cocoon ( Fig. 21, 22 View FIGURES 21 – 25 ): slipper shaped; of markedly thickened dark brown silk threads, loosely woven; inner pocket more closely woven than remainder of cocoon, with distinct anterior edge and occasional indication of dorsomedial projection; lateral extremities of cocoon either with distinct edge, or individual anchor points for silk threads.
Larva (last instar).
Body ( Fig. 29 View FIGURES 27 – 31 ): total length 4.3–4.5 mm. Head ( Fig. 30 View FIGURES 27 – 31 ): markedly bicoloured; length 0.51–0.65 mm, width 0.54– 0.55; distance between antennal bases 0.36–0.38 mm; lateral margins of head smoothly convex; cephalic apotome translucent and pale yellow, median head spots negative, surrounded by rich brown pigmentation, producing marked figure-of-8 shape; cuticle slightly corrugated, no extra vestiture beyond normal array of setae; ecdysial lines narrowed slightly anterior of stemmata, expanded posteriorly to curve broadly posteriorly; cervical sclerites, separate, not markedly developed, poorly pigmented. Antenna ( Fig. 32 View FIGURES 32 – 37 ): articles evenly light brown, medial article slightly paler; 0.35 mm total length, extended just beyond apex of fan stalk; ratio of length of apical, medial and basal articles 1:1.6:1.2, length of basal article 4.8 times width. Labral fan: stalk clear and relatively short; 40–46 short, substantial rays, 0.56 mm in length, 0.012 mm in width at mid length; distinct pattern of microtrichia, longer microtrichia 0.8 times longer than ray width, interspersed with ca. 8 smaller microtrichia. Postgenal cleft ( Fig. 33 View FIGURES 32 – 37 ): markedly rounded, posterior tentorial pit region not markedly sclerotized and pigmented. Postgenal bridge: subequal in length to hypostoma, slightly paler than dark genae, elongated posteroventral muscles spots not visible. Hypostoma ( Fig. 34 View FIGURES 32 – 37 ): 11 teeth, median tooth shorter than lateral teeth, the latter broadly based, sublateral teeth small and directed laterally; one paralateral tooth; 5–6 small but distinct lateral serrations; 5 hypostomal setae per side, subparallel to edge of hypostoma. Mandible ( Fig. 35, 36 View FIGURES 32 – 37 ): normal development, brushes not markedly produced; outer teeth poorly developed; apical tooth well developed; three subapical teeth not substantial decreased in size proximally and then 5–6 spinous teeth (markedly few); serration well developed and sensillum distinct; blade region elongated and smooth. Thorax: wider than anterior abdomen, markedly pale dorsally, pale yellowish brown otherwise; pharate pupal gill histoblast ( Fig. 31 View FIGURES 27 – 31 ) with filaments in broad L-shape; directed ventrally then curved posteriorly to then curve dorsally again and coil back towards the gill base. Abdomen: clear pale yellowish grey, intersegmental regions pale producing slightly banded appearance; abdominal segments I–IV narrowed, expanded laterally at 5th segment—producing a slight amphora shape, but not ventrally; ventral tubercles poorly developed and rounded; cuticle with minute but distinct simple setae, more dense on posterior segments ( Fig. 37 View FIGURES 32 – 37 ). Anal sclerite: anterior and posterior arms short, robust, both abruptly tapered; accessory sclerites small, distinct and sometimes comma-shaped with the tail directed ventrally ( Fig. 29 View FIGURES 27 – 31 , 37 View FIGURES 32 – 37 ). Rectal scales present and interspersed with the abdominal setae ( Fig. 38 View FIGURE 38 – 40 ). Rectal papillae: three simple lobes with rarely a small basal lobe on the lateral papillae. Posterior proleg circlet of hooks ( Fig. 29 View FIGURES 27 – 31 , 37 View FIGURES 32 – 37 ): directed posteriorly; 75–80 rows of hooks, 12–14 per row (total ca. 1 000).
Etymology: Not specifically mentioned by Pomeroy, but implicitly for William Mansfield Aders (Crosskey and Davies 2012).
Material examined: Full array of eggs, larvae, pupae, male and female adults.
Mayotte, Comoro Archipelago. Ouroveni, 2-x-2008, S12.80831° E45.12761°; Bouyouni, 5-x-2008, S12.74036° E45.14258°; Batirini, 5-x-2008, S12.76047° E45.11183°; Coconi, 6-x-2008, S12.83481° E45.12827°; Combani, 7-x-2008, S12.77144° E45.14753°; Koualé, 8-x-2008, S12.80542° E45.16381°; Chirini, 8-x-2008, S12.77769° E45.10456°; Koualé, 9-x-2008, S12.79714° E45.18572°. All collected by N. M-S.
Deposited in the Strickland Museum, Department of Biological Sciences, University of Alberta, Edmonton, CANADA. Other material is in the personal collection of N. M-S.
Comments. Below we discuss differences between the original descriptions by Pomeroy (1922), Gibbins (1934), Freeman and de Meillon (1953), Crosskey (1960), Rubtsov (1962) and Crosskey (1969) of African mainland S. adersi and the Mayotte material as given above.
Adults. The Mayotte adults of S. adersi show little difference to previous descriptions of the species. A character, however, apparently not noted elsewhere is the distinct pigmented region around the campaniform sensory organs on the wing at the junction of the radial sector (Rs) vein and 1st radial (R1) vein ( Fig. 8 View FIGURES 8 – 13 ).
We make the point, already known, that the mandible of the female has teeth only on one side ( Fig. 4 View FIGURES 2 – 7 ). This was described by Freeman and de Meillon (1953: 111) who described 18 small teeth on the inner surface only. The Mayotte material has 16. It is perhaps surprising that more importance has not been assigned to this character state. We comment because in simuliids, teeth on both sides of the mandible is plesiomorphic. The one-sided condition is known for Austrosimulium (Craig et al. 2012) where it is a diagnostic character for that genus in the Australasian region. Further, for Austrosimulium it is known to be a derived character—reverting to a two-sided condition in gynandromorphs (Craig and Crosby 2008). Otherwise teeth on one side of the mandible is sporadic in Simuliidae (e.g., Gomphostilbia, Takaoka and Davies 1995 , Bentinck 1955). Is it possible that a one-sided mandible is diagnostic for Meilloniellum? We did not investigate further.
The lacinia in the Mayotte material has reduced numbers of recurved teeth with only 5 inner and 12 outer teeth ( Fig. 6 View FIGURES 2 – 7 ). For other S. adersi material, Freeman and de Meillon (1953) describe 9 and 13 teeth respectively
The rather finely crafted mandible and reduced number of teeth on the lacinia along with the relatively short mouthparts tend to indicate that S. adersi would be a bird feeder—in agreement with the similar mouthparts in Austrosimulium . Bird feeding in S. adersi is well confirmed, but such mouthparts do not preclude feeding on humans and other mammals, as this simuliid is known to do readily (Gibbins 1934, Bequaert 1939, Palmer and de Moor 1998).
Simulium adersi is the only species of Meilloniellum that possesses a haired pleural membrane (Crosskey 1969). Of fine nature this vestiture is difficult to observe and also the hairs dislodge easily, usually leaving only the sockets visible ( Fig. 12 View FIGURES 8 – 13 ).
There is little difference in adult legs between localities. Mayotte females, however, have a calcipala ( Fig. 10 View FIGURES 8 – 13 ) that is slightly more elongate that that illustrated by Gibbins (1934; his fig. 2e) and the membranous area of the pedisulcus less extensive.
Crosskey (1969) described the abdomen of the Meilloniellum species as thickly and evenly covered with pale scales. Freeman and de Meillon (1953) describe the abdomen as black with dense silvery and yellow scales. The Mayotte material is not black and while there are pale scales, these are sporadic. However, the available females and males give every impression of having been worn and they perhaps are not showing the original vestiture.
Pupae. Difference between pupae of Mayotte S. adersi and those from elsewhere show in the gills and the cocoon. The cocoon as first illustrated by Gibbins (1934; his fig. 8) shows it to be apparently of close weave. Another illustration by Freeman and de Meillon (1953; their fig. 32h) shows the weave to be more open—similar to the Mayotte material ( Fig. 21 View FIGURES 21 – 25 ). Not mentioned, or illustrated previously, is the internal pouch shown clearly here ( Fig. 22 View FIGURES 21 – 25 ). The gills of the Mayotte pupae have the same number of filaments and branching pattern, but are noticeable longer overall, with relatively shorter ventral filaments. Gibbins (1934; his fig. 7: 1936; his fig 5a), shows the total length of the gill to be ca. 1.2 mm. with the ventral filaments slightly shorter than the others, as does Crosskey (1969; his fig. 194). The Mayotte material has gill length of ca. 2.1 mm ( Fig. 23 View FIGURES 21 – 25 ).
Larvae. Gibbins (1934; his fig. 5a) specifically illustrated the cephalic cuticle of the larva as armed with minute spines, and mentioned a pigmented area below the stemmata as also spined. Such has not been commented on by following workers and the Mayotte material has merely normal chaetotaxy ( Fig. 30, 31 View FIGURES 27 – 31 ) as detailed for simuliids by Craig (2005). Pigmentation pattern of the head, namely the negative head-spots is similar for all material (Crosskey 1960; his fig 21), however the trident-shaped lateral pigmentation posterior to the stemmata is overall slightly darker in the Mayotte material. The rendition of the lateral head in Crosskey's figure appears to show the spined region noted by Gibbins (1934).
Aspects of the ventral head capsule of the larvae are similar in all material. Shape of the postgenal cleft and postgenal bridge (Crosskey 1960; his fig. 38: 1969; his fig 250) agree with the Mayotte material ( Fig. 33 View FIGURES 32 – 37 ). However, that of Mayotte have hypostomal teeth ( Fig. 34 View FIGURES 32 – 37 ) not as prominent as otherwise illustrated (Gibbins 1934; his fig. 6: Crosskey 1960; his fig 57). In particular the median tooth does not protrude beyond the lateral teeth.
Crosskey (1960) described the larval antenna of S. adersi as having the basal article (his first segment) 6.0 times as long as broad. That of the Mayotte material is only 4.8 times as long as broad. Further, Crosskey (loc cit.; his figure 79) gave proportions of the three articles as 1.0:1.25:1.28, those for the Mayotte material are 1.0:1.47:1.18 ( Fig. 32 View FIGURES 32 – 37 ) where the median article is relatively longer.
Labral fan ray number is variable. Gibbins (1934) stated that there were 28–32, but Crosskey's (1960) reexamination of type material showed there to actually be 38–41. Overall, Crosskey (loc. cit.) reported 38–47 rays, with 44 the mean. The Mayotte material has 40–46 rays. Whether the minor difference in number it taxonomically useful is moot. Fan ray number is known to be phenotypically plastic—dependent on nutrition and velocity of water (Zhang 2006).
Crosskey (1960; his fig. 90) illustrated three basic conditions of larval mandible for African simuliids. That of S. adersi were of the more common condition ( Fig. 35 View FIGURES 32 – 37 ). For Mayotte material, however, the mandibular serration and sensillum are larger and more closely applied ( Fig. 36 View FIGURES 32 – 37 ) than illustrated by Crosskey (loc cit.; his fig 104). Gibbins (1934; his fig. 5d) illustration appears somewhat stylized, and the mandibular sensillum is rendered as markedly small and the serration smaller too.
Crosskey (1960; his fig 126) illustrated the maxillary palpus and described it as 3.1 time as long as the basal width, not including the apical sensilla. The palpus of the Mayotte larvae is only 2.5 times as long as the basal width.
Overall shape of the posterior abdomen and relative size of the ventral tubercles of Mayotte S. adersi last instar larvae agrees closely with the illustration by Crosskey (1960; his fig. 157), but that illustration lacked any accessory sclerite. The anal sclerite is very similar ( Fig. 37 View FIGURES 32 – 37 ) to that shown by Crosskey (loc. cit.; his fig 164), but again his material lacked the accessory sclerite(s). Never-the-less, Crosskey (1969) stated that there are accessory sclerites as "sometimes only minute weakly sclerotized trace". Mayotte material has a small, but distinct, accessory sclerite on each side ( Fig. 37 View FIGURES 32 – 37 ).
Rectal scales were not mentioned by Gibbins (1934, his fig. 5c) and are notably absent from the illustration. Rubtsov (1962), in his key to larvae, divided Meilloniellum species into those with and without rectal scales. Crosskey (1969) in his revision of Meilloniellum has rectal scales as a diagnostic character for the subgenus. The Mayotte larvae clearly possess these structures ( Fig. 37 View FIGURES 32 – 37 , 38 View FIGURE 38 – 40 )
Gibbins (1934) describes the rectal papillae as trilobed and each with smaller secondary appendages. Freeman and de Meillon (1953) merely refer to S. adersi rectal papillae as trifid—there is no mention of lateral lobes. Rubtsov (1962) describes Meilloniellum with branched rectal papillae. Crosskey (1960) describes the papillae as having several secondary lobes each. The Mayotte material are basically simple trifid structures with, very rarely, a slight indication of secondary lobules (poorly visible in Fig. 29 View FIGURES 27 – 31 ).
The number of hooks comprising the circlet of hooks on the posterior proleg is variable. Gibbins (1934) stated there were ca. 120 rows of 19–25 hooks for the Nsadzi larvae. Crosskey (1960) gave a much lower number of 69– 75 rows with 9–15 hooks. The Mayotte larvae have some 75–80 rows of 12–14 hooks. These characters states are phenotypically plastic, and Palmer and Craig (2000) showed a clear correlation between velocity of water in the habitat of the larva and total number of hooks. Of relevance here too is that the original larval material of S. adersi from Nsadzi Island in Lake Victoria were larger (5.8–6.0 mm in length. Gibbins 1934) than other known larvae (Crosskey 1960. 4.5–5.0 mm in length). The Mayotte larvae are of the latter size.
Overall, the disagreement in morphological character states between Mayotte S. adersi and those from the African mainland, suggest that the Mayotte entity is a separate species. Before, however, that is formally done, the material needs to be compared with S. adersi from Madagascar. Indeed, the whole complex needs further examination, including using molecular and cytological methods.
General biology of Simulium adersi
Commonly mentioned in the literature regarding S. adersi is that the immature stages have a remarkable range of habitat. Gibbins (1934) commented about larvae being found on the wave-swept shore of islands in Lake Victoria, in particular Nsadzi Island—a most unusual habitat for simuliid larvae. Immature stages have been found in almost every type of running water, from large rivers to small runnels, high conductivity, estuarine (Crosskey 1960), and less so, high quality water (Palmer and de Moor 1998; de Moor 2003). Oddly, because of that latter aspect, S. adersi has been investigated as a possible indicator of water quality in the Buffalo River, South Africa (Palmer et al. 1996).
Of economic importance was replacement of S. adersi and S. nigritarse , both relatively non-pest species, with the seriously biting S. chutteri as a result of interbasin water transfer projects involving the Vaal, Orange and Great Fish rivers (e.g., O'Keeffe and de Moor 1988). Effects of these projects are still of considerable interest (Rivers- Moore et al. 2007, Gupta and van der Zaag 2008, de Beer and Green 2012).
While providing considerable details about water quality, velocity, substrates, and density of aquatic insects, Starmühler (1976, 1979) did not identify Simuliidae except to Family, so nothing can be said regarding ecological requirements of S. adersi on Anjouan. Considerable ecological data on the streams sampled in Mayotte was compiled during the Surveillance Monitoring programme in 2008 and 2009. Most localities were forested in one manner or other (e.g., Fig. 39, 40 View FIGURE 38 – 40 ). For the localities from which we examined material, larvae of S. adersi were normally found on vegetation in situations where the water velocity was fast. No exact velocity measurements were made. Most sites had substrates of cobble or boulders (e.g., Fig. 39 View FIGURE 38 – 40 ), but occasionally solid rock (e.g., Fig 40 View FIGURE 38 – 40 ). Water temperatures ranged from 22–31°C, conductivity from 129–394 µS/cm and oxygen saturation 21–93%. Water was generally clear, but there was considerable anthropogenic impact from washing, excrement and garbage. Details of all sites can be obtained directly from N. M-S.
There appears to be no information on nuisance value of S. adersi in Mayotte. Certainly no biting was recorded by people involved in the Surveillance Monitoring programme.
Palmer and de Moor (1998) report that S. adersi females lay some 400 eggs, considerably more than for the single example of Mayotte S. adersi . Furthermore, the eggs were larger. Perhaps this indicates that the Mayotte female had not blood-fed, as suggested by observations by N. M-S. on lack of biting. Oviposition by S. adersi takes place underwater (Gillet and Lebied 1959, Balay 1964, Crosskey 1969) with eggs clustered on hard substrates. Laboratory studies show that the eggs take 13 days to hatch at ca. 25°C and the larvae a minimum of 17 days to pupae at the same temperature (Begemann 1980). Seven larval instars were convincingly demonstrated for S. adersi by Elouard (1987)—in full agreement with the modal number known for Simuliidae (Adler et al. 2004) .
Parasitoids are known for larvae of S. adersi ; two genera, Caudospora and Weiseria spp. were reported by Jamnback (1970).
Female adults S. adersi are primarily bird feeders and a pest of poultry, but are well known to bite sheep, goats and humans (Bequaert 1939). On humans the neck, head and ears are preferred. Fallis and Raybold (1975) convincingly showed that S. adersi females were attracted to carbon dioxide exhalations and not silhouettes alone of animals. Oddly, even recently, S. adersi was still referred to as non-anthropophilic (e.g., Mbah et al. 2003),
Simulium adersi females are known vectors of haematozoa in poultry and transmission of Leucocytozoon neavei and Leucocytozoon schoutedeni was investigated by Fallis et al. (1973a). Transmission of Trypanosma numidae from S. adersi to birds was also experimentally demonstrated by Fallis et al. (1973b). Because of the nuisance value of that biting, and the transmission of haematozooans, S. adersi has been the subject of control proceedings (e.g., Car 1984, Myburgh and Neville 2010), using the organophosphate Temophos™ and Bacillus thuringiensis israelensis (Bti) . Similarly, because of the possibility that S. adersi might be involved in vectoring Onchocerca volvulus , the causative agent of onchocerciasis, S. adersi was early involved in research related to that disease (e.g., Wanson 1950). Simulium adersi could be a potential vector of O. volvulus (Wegesa 1970) and is still considered so (e.g., Lamontellerie 1963, Johnson et al. 1982, Roberts and Irving-Bell 1996).
Biogeography
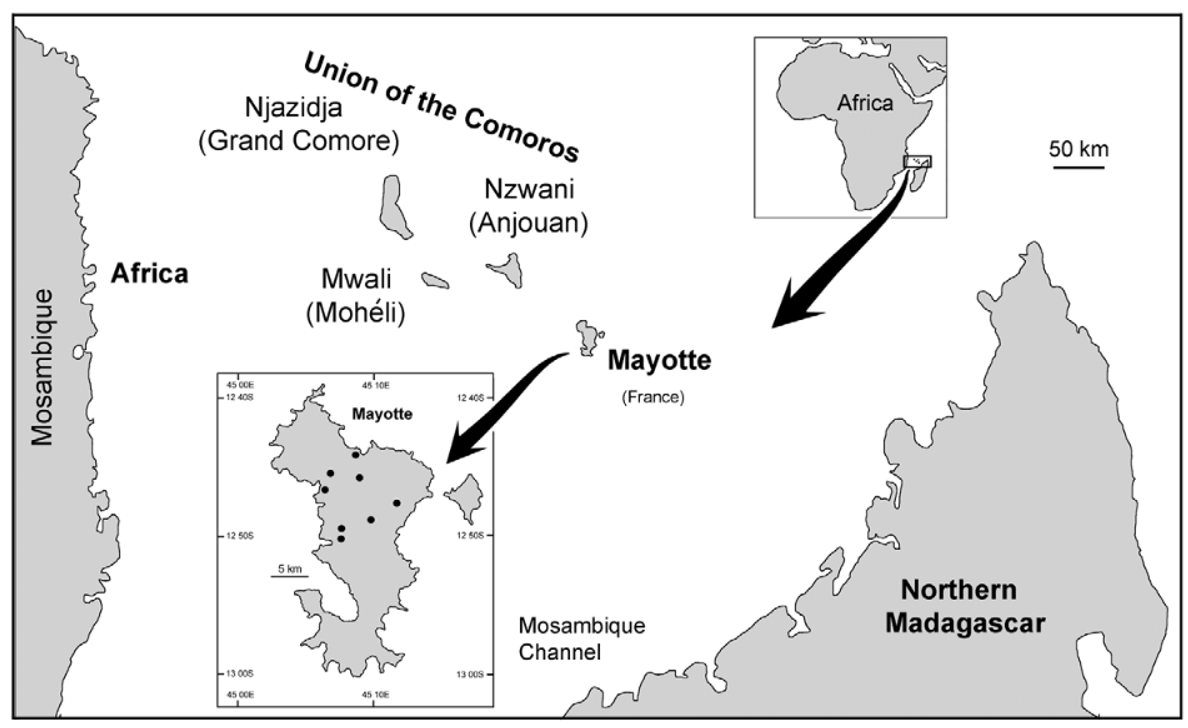
There has been much written about biota of hot-spot islands, in particular that of Hawai'i (e.g., Wagner and Funk 1995; Losos and Rickleffs 2010). A parsimonious explanation for insect biota of such islands is by wind dispersal and this is the explanation suggested for Simuliidae on islands in Polynesia, and the Gulf of Guinea. (e.g., Craig 2003, Mustapha et al. 2006). For the Comoros, wind patterns could indicate an origin from the African mainland. From October to April there is a wet season (Kashkasi) with predominantly strong, consistent north to northwesterly monsoon winds off the Indian Ocean. A dryer season (Kusi) is from May to September with less intense southerly winds.
Given that the Comoro islands are situated essentially midway between Mozambique on the African mainland and northern Madagascar ( Fig. 1 View FIGURE 1 ), such wind dispersal out of Africa to the west would not be unreasonable, but S. adersi occurs in Madagascar (Pilaka and Elouard 1999) and that cannot be ignored as the possible source population. Furthermore if presence of S. (Xenosimulium) imerinae on Mohéli (Adler and Crosskey 2013; Crosskey 2012) can be confirmed, such would indicate that origin of Comoro simuliids was more likely that of Madagascar. The subgenus Xenosimulium is endemic to Madagascar (Adler and Crosskey 2013).
Simulium adersi is widespread in Madagascar (Pilaka and Elouard 1999). While Elouard and Pilaka (2001; their Fig. b) provide keys and distribution maps of Madagascan simuliids and list some 27 species from the island, detailed description of S. adersi from there is not yet available, so resolving the question of origin of the Mayotte S. adersi will require that examination. With S. adersi on both Mayotte and Anjouan, an expectation might be that the two simuliid faunas would be closely related. It could be though, that the islands were colonized from separate sources, or dispersal events (see below). Nothing, however, is known about long range dispersal ability of this species.
Other species known for Comoro islands (Crosskey 2012) only partially help with historical biogeography of simuliids of the archipelago. Simulium ruficorne is markedly widespread in the region, occurring in Africa, the Comoros, Madagascar, and elsewhere, so gives no indication of origin. However, Simulium dentulosum , widespread in Africa is apparently absent from Madagascar (Elouard and Pilaka 2001), so this indicates dispersal from the west to Anjouan, at least for that species.
The Comoro appears typical of hot-spot island arrays. Geologically, the western island of Grand Comore is the youngest and still is volcanically active at Karthala volcano; ages increase eastwards through Mohéli, Anjouan, with Mayotte the oldest island. The lineage appears to have been formed as the Somali Plate drifted over a magma hot spot at ca. 50–70 mm / yr. (Hajash and Armstrong 1972; Emerick and Duncan 1982). They estimated for Mayotte that shield building began 5.4 million years ago, with post erosional magma from 3.8–2.4 million year ago and then later flows at 1.5 million. Anjouan had only one date known and that was for 1.2 million years ago. Mohéli commenced shield building at 2.8 million years ago, with other flows at 1.9–1.6 million and more recently at 0.8–0.7. Shield-building of Grand Comore started merely 0.1 million years ago.
Mayotte appears to have followed the basic formation pattern known for Polynesian hot spot islands (Duncan and McDougall 1976; Duncan et al. 1994; Craig 2003), where, following shield-building volcanics, erosion allows further magma eruptions, often at regular intervals. Later there can be 'rejuvenating flows' of magma, but eventually the island becomes submerged and forms a guyot. In the Nosy region, northwestern Madagascar, volcanics are dated at 10 million years old. Bathymetry shows flat-topped submerged sea-mountains (guyots), the Banc du Geyser, between the Nosy region (NW Madagascar) and Mayotte ( Taiwan Ocean Research Institute URL—http://cmtt.tori.org.tw/)—consistent with islands forming prior to that of Mayotte, which as such may have provided stepping stones for biota to reach the present Comoro islands.
However, Nougier et al. (1986), in a detailed review of geochronology of the archipelago suggest that Mayotte was ca. 8 million years of age, with Anjouan 11.1–3.9 mya, Moheil 5.0–3.2 mya, and Grande Comore perhaps 0.01–0.13 mya. They also question the hot spot hypothesis for formation of the array of islands; suggesting instead that volcanism of the Comoros was controlled by old and deep lithospheric fractures that have been reactivated at different times.
Perhaps pertinent to origin of simuliids on Comoro islands is an in-depth study of dispersal of frogs in the Indian Ocean region and in particular those of Mayotte (Vences et al. 2003). These frogs require fresh water. Phylogenetic analysis involving calibration from geological, morphological and molecular data indicates that a Boophilis and a Mantidactylus species, found only on Mayotte, dispersed from Madagascar in independent transmarine events less than 8.7 my ago. For simuliids an expectation might be an age less than that, since permanent running water takes time to develop (Craig 2003).
With the rates of divergence now known for simuliids on Tahiti (Joy et al. 2007) and New Zealand (Craig et al. 2012), the probable ages of Mayotte give ample time for allopatric divergence of S. adersi from its precursor, either from the African mainland, or Madagascar.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |