Scaptomyza Hardy, 1850
|
publication ID |
https://doi.org/10.11646/zootaxa.159.1.1 |
|
DOI |
https://doi.org/10.5281/zenodo.5093490 |
|
persistent identifier |
https://treatment.plazi.org/id/03D287A0-EB25-E048-BE48-F9D4BBD3FE17 |
|
treatment provided by |
Felipe |
|
scientific name |
Scaptomyza Hardy, 1850 |
| status |
|
Scaptomyza Hardy, 1850 View in CoL View at ENA
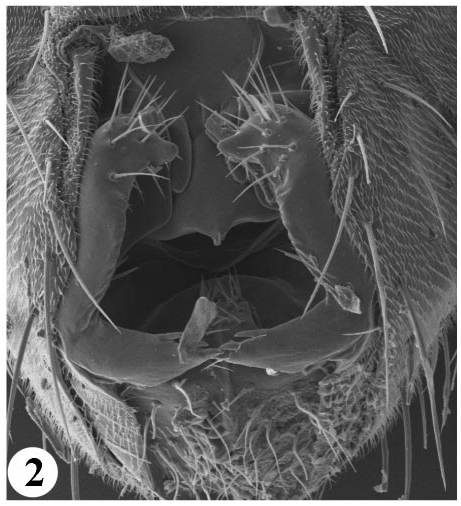
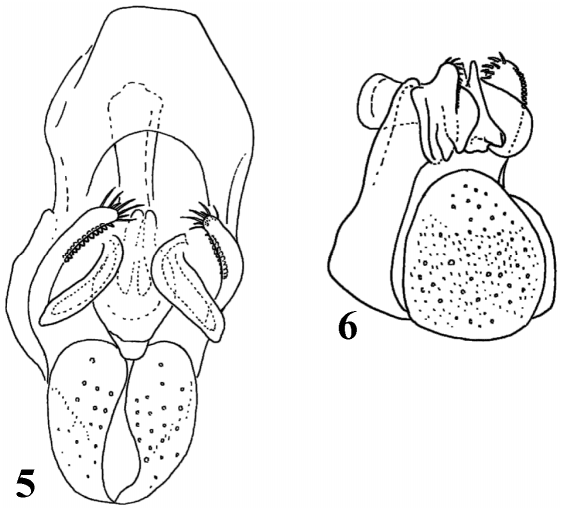
( Figures 1—6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURES 56. 5 )
Scaptomyza Hardy, 1850: 361 View in CoL . Type species: Drosophila graminum Fallén, 1823 View in CoL , by subsequent designation ( Coquillett, 1910: 603).
Titanochaeta Knab, 1914: 167 . Type species: Titanochaeta ichneumon Knab, 1914 , by original designation. Syn. nov.
Scaptomyzella Hendel, 1928:290 . Type species: Drosophila flava Fallén, 1823 View in CoL , by original designation.
Scaptomyzetta Hendel, 1928: 290 (incorrect original spelling of Scaptomyzella ).
Grimshawomyia Hardy, 1965: 535 . Type species: Drosophila perkinsi Grimshaw, 1901 View in CoL , by original designation. Syn. nov.
Engiscaptomyza Kaneshiro, 1969: 80 (as subgenus of Drosophila View in CoL ). Type species: Drosophila crassifemur Grimshaw, 1901 View in CoL , by original designation. Syn. nov.
Diagnosis. Scaptomyza is a cosmopolitan genus that currently contains about 15 subgenera ( Wheeler 1981, 1986), some of which have been designated as separate genera at one time or another ( Frey 1954; Hackman 1959, 1982; Malloch 1934). The traditional definition of Scaptomyza includes taxa with two to four rows of acrostichal setulae, two pairs of postsutural dorsocentral setae (and sometimes with a single set of presutural dorsocentrals as well), the third costal section 2.5 times longer than the fourth, and the head distinctly longer than high ( Hardy 1965). However, a rather large radiation of about 150 described species present in the Hawaiian Archipelago, has broadened this definition somewhat, mainly because of atypical characters possessed by some of these taxa ( Hackman 1959, 1962, 1982). For example, many members of the subgenus Elmomyza have six rows of acrostichal setulae, suggesting that this character may be quite variable within Scaptomyza . Therefore, having either two or four rows of acrostichals is not a good synapomorphy for the genus Scaptomyza , although it may be useful at delimiting some subgenera.
Perhaps the best character defining all Scaptomyza is the presence of well developed, exposed surstyli and enlarged lobes on either the epandrium (ninth tergite), cerci, or both. These morphologies are also characteristic of the genera Grimshawomyia and Titanochaeta , as well as the subgenus Engiscaptomyza . An additional character, found in females of most species, is a weakly developed, fleshy, nondentate ovipositor. Titanochaeta is atypical in this character as females of this group have a slender, sharply pointed, styletlike ovipositor, a character that may be an adaptation to a lifestyle as a spider egg sac predator.
Methods. We have examined the types, as well as large series of other material, from all species placed in Titanochaeta , Engiscaptomyza , and Grimshawomyia ( Table 1 View TABLE 1 ). We also have examined material from most recognized subgenera of the genus Scaptomyza . Based on this work, we selected a number of taxa placed in the genus Scaptomyza , as well as representatives of Drosophila ( Engiscaptomyza) , Grimshawomyia , and Titanochaeta thought to be closely related to this genus, for use in the current molecular and morphological analyses. Over 3.3 kilobase pairs of nucleotide sequence from five genes (16S, Adh, COI, COII, Gpdh) were examined in about 120 drosophilid species using a variety of phylogenetic methods ( Bonacum 2001). The phylogeny shown in figure 1 is the result of a maximum parsimony analysis (addition sequences = random, number of replicates = 100, branch swap = TBR). The search recovered four most parsimonious trees [length 33,181; CI = 0.31; RI = 0.53; see Bonacum (2001) for more detail]; figure 1 is from the strict consensus. Measures of support include bootstrap proportions (BP; Felsenstein 1985, 1988), and decay indices (DI; Bremer 1988). This phylogeny shown is part of a larger study treating phylogenetic relationships within the entire Hawaiian Drosophilidae ( Bonacum 2001; Bonacum et al. in press) and includes several outgroups, as well as representatives of all major Hawaiian Drosophila lineages. Based on this taxon sampling, we feel confident in making statements concerning the relationships of the Hawaiian Drosophilidae and the genus Scaptomyza .
Morphological analyses were done either using light or scanning electron microscopy. Specimens were prepared as follows: adult flies stored in 70% ETOH were completely dehydrated via sequential washes with 80%, 90%, 95% and 100% ETOH. These specimens were then critical point dried using standard protocols ( Grimaldi 1987). Male genitalic structures were dissected from the abdomen and adhered to a specimen mount (Ted Pella, Inc.) using double coated, carbon conductive tabs (Ted Pella, Inc.). The material was sputter coated and visualized using a Hitachi S4700 Field Emission Scanning Electron Microscope. All image files were saved in.tif file format and edited in Adobe Photoshop 5.0 (Adobe Systems, Inc.). Image files are available upon request.
Results and Discussion. The molecular and morphological data strongly support the notion that the genera Titanochaeta and Grimshawomyia , as well as the Drosophila subgenus Engiscaptomyza , actually belong within the genus Scaptomyza . The molecular phylogeny we present ( Fig. 1 View FIGURE 1 ) shows high support for a clade containing these three endemic Hawaiian groups with several subgenera of the genus Scaptomyza (BP = 100, DI = 24.75). Although support for the Hawaiian Scaptomyza lineage plus Titanochaeta , Grimshawomyia and the subgenus Engiscaptomyza is quite robust, relationships within this clade are not well supported. Only the monophyly of the subgenus Bunostoma (BP = 100, DI = 44.5) and the sister group relationship of S. (Scaptomyza) graminum and S. (Parascaptomyza) elmoi (BP = 100, DI = 24) are well supported ( Fig. 1 View FIGURE 1 ). The latter relationship, however, implies that the subgenus Parascaptomyza is not monophyletic. This phylogeny also calls into question the monophyly of Engiscaptomyza , placing S. crassifemur as the sistertaxon of S. chauliodon and S. nasalis as the sister of S. palata ( Fig. 1 View FIGURE 1 ). Clearly, the large Scaptomyza lineage will need to be surveyed more extensively and completely revised in order to resolve these issues..
Scanning electron microscopy was used to compare the morphology of the male genitalia of Titanochaeta , Grimshawomyia , and the crassifemur group with Scaptomyza and Drosophila . It is clear that, based on several characters, the three endemic Hawaiian groups are more closely related to Scaptomyza than they are to Drosophila . For example, the epandria and cerci of Titanochaeta , Grimshawomyia , Scaptomyza , and Engiscaptomyza are all highly modified, possessing expanded lateral lobes that often bear elongate setae ( Figs. 2–6 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURES 56. 5 ). Hardy (1965: 606) noticed these characters and cautioned against referring to them as secondary claspers because he preferred “to use this term only for those distinctly clasperlike lobes...which bear strong spines.” In addition, the genitalia of Scaptomyza , Titanochaeta , Grimshawomyia , and Engiscaptomyza have a more “open” arrangement, where the surstyli and lateral lobes of the epandrium form a “cup” on the ventrodistal surface of the abdomen ( Figs. 26 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURES 56. 5 ). This is in contrast to the genus Drosophila , where the surstyli are closely oppressed on either side of the aedeagus and lateral lobes on the epandrium or anal plates are generally absent.
Chromosome studies also suggest a close affinity between Scaptomyza , Engiscaptomyza and Titanochaeta ( Clayton et al. 1972; Yoon et al. 1975). The metaphase configurations (1 Vshaped, 3 rods, and 1 dot; N = 5) is shared between Scaptomyza , Titanochaeta , Engiscaptomyza and some species in the modified mouthparts species group (genus Drosophila ). All other Hawaiian Drosophila species have the “ancestral” karyotype (5 rods and 1 dot; N = 6) for the genus Drosophila . It has been suggested that this reduction in chromosome number has taken place via centric fusion events ( Patterson & Stone 1952). Our molecular phylogeny ( Fig. 1 View FIGURE 1 ) suggests that this has taken place at least twice – once in the modified mouthpart species and again in the Scaptomyza lineage (which contains Titanochaeta , Engiscaptomyza , and Grimshawomyia ).
Based on the morphological, chromosomal, and molecular evidence, we propose placing the genera Titanochaeta and Grimshawomyia into the genus Scaptomyza as subgenera. We are also moving the subgenus Engiscaptomyza from the genus Drosophila to Scaptomyza. This placement will broaden the morphological concept of the genus Scaptomyza which will, in turn facilitate further taxonomic studies on this poorly understood and complex group.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Scaptomyza Hardy, 1850
| O’Grady, Patrick, Bonacum, James, Desalle, R. & Val, Francisca Do 2003 |
Engiscaptomyza
| Kaneshiro, K. Y. 1969: 80 |
Grimshawomyia
| Hardy, D. E. 1965: 535 |
Scaptomyzella
| Hendel, F. 1928: 290 |
Scaptomyzetta
| Hendel, F. 1928: 290 |
Titanochaeta
| Knab, F. 1914: 167 |
Scaptomyza
| Coquillett, D. W. 1910: 603 |