Millepora laboreli, Amaral, Fernanda M. D., Steiner, Andrea Q., Broadhurst, Matt K. & Cairns, Stephen D., 2008
|
publication ID |
https://doi.org/10.5281/zenodo.184834 |
|
DOI |
https://doi.org/10.5281/zenodo.5616268 |
|
persistent identifier |
https://treatment.plazi.org/id/03D287D5-FF9F-271B-4A9B-FACC402A7B55 |
|
treatment provided by |
Plazi |
|
scientific name |
Millepora laboreli |
| status |
|
Millepora nitida Verrill, 1868 View in CoL
Verrill (1868) described this species as having a “corallum forming low rounded clumps, four to six inches high, consisting of short, rapidly forking, rounded or slightly compressed branches” and Boschma (1962) stated that M. nitida is easily distinguishable from other species of Millepora of the western Atlantic Ocean. Amaral (1997) and Amaral et al. (2002) observed that M. nitida occurs predominantly in ramified form, although hemispheric and honeycombed forms also occur. Most colonies are smooth in texture, with few commensal cirripeds ( Table 1 View TABLE 1 ). Amaral et al. (2002) showed that M. nitida had shallow and isolated ampullae, mean diameters of gastropores and dactylopores comparable to M. braziliensis and a similar mean ratio of dactylopores per gastropore ( Table 2 View TABLE 2 ). Unlike M. alcicornis and M. braziliensis , the form of the pores was predominantly irregular ( Table 1 View TABLE 1 ). Compared to all other species examined, M. nitida showed a greater transverse diameter, an intermediate colony height, and had greater and fewer mean numbers of cyclosystems and gastropores per cm2, respectively ( Amaral et al. 2002).
Millepora sp. a Amaral et al. 2002
Type material: BRAZIL, Manuel Luiz Marine State Park, State of Maranhão ( type locality), 1998, 30 m, collector Marco Hudson ( holotype, Cnidarian Collection of the Laboratório de Ambientes Recifais da Universidade Federal Rural de Pernambuco, LAR/ UFRPE nº 0 95 ( Fig. 2 View FIGURE 2 ); 10 paratypes. One paratype (number 02957) has been deposited at the National Museum in Rio de Janeiro.
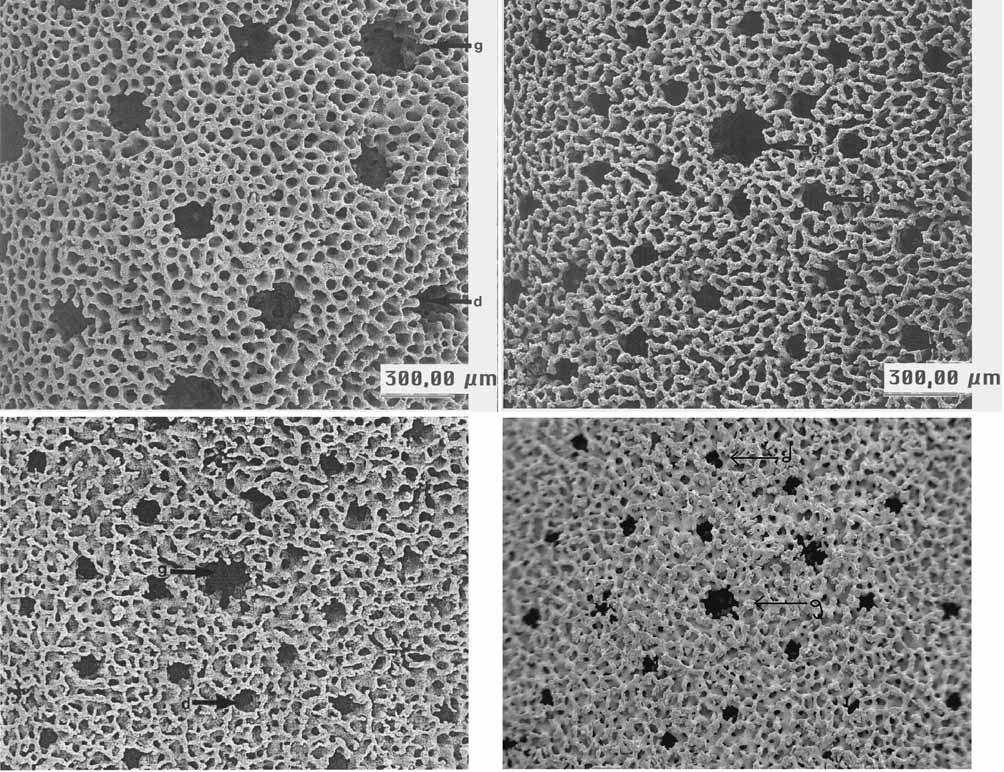
Description (based on 11 samples—Figs. 3 and 4): Brown in color, growing in the form of pillars or fans at a depth of 30–32 m, with a single principal branch. Growth form always columnar (average height of 162.7 mm ± 15.5 SE—higher in the field), with a smoother and flatter texture than the other species of Millepora . Transverse of 83.8 mm (± 12). Mean gastropore diameter is 0.11 mm (± 0.004), gastropore density is 23.3 per cm2 (± 2.50) and dactylopore diameter is 0.08 mm (± 0.002) with 6.30 (± 0.40) dactylopores and 1.45 (± 0.21) cyclosystems per gastropore. Mean ampullae diameter is 0.36 mm (± 0.03).
Diagnosis of the holotype. The holotype’s growth form is columnar, with two smooth projections and an irregularly textured base. It is 265 mm high, with the secondary projection measuring 145 mm. The transverse diameter is 205.6 mm. The average gastropore diameter and density are 0.3 mm and 20.67 per cm2, respectively. Dactylopore diameter is 0.09 mm, with 6.38 dactylopores and 1 cyclosystem per gastropore. Ampullae diameter is 1.1 mm. Dactylopores and gastropores are deformed and very shallow.
Etymology. This species’ name honors Dr. Jacques Laborel (retired professor of the Université de Aix- Marseille, France) for his pioneering work and great contribution to the knowledge of Brazilian reefs.
Diagnosis of the species ( Figs. 5–6 View FIGURE 5 View FIGURE 6 ). Millepora laboreli can be identified from all other specimens of Millepora due to its restricted distribution (it only occurs in the Manuel Luiz Coral Banks, differently from the other species which have a wider distribution within Brazil and/or the tropics); its always columnar growth form, sometimes with projections (vs. variable forms); its very smooth texture (vs. more irregular surfaces); and its reduced number of ampullae (approximately one vs. five to thirteen per colony). Overall, the characters of M. laboreli are smaller than those of the other Brazilian Millepora species (diameter of ampullae, dactylopores, and gastropores) and there are fewer ampullae ( Figs 5–6 View FIGURE 5 View FIGURE 6 ). Specifically in relation to the other Millepora species, M. laboreli can be distinguished from M. alcicornis and M. braziliensis because of its small, deformed and extremely shallow dactylopores and gastropores (vs. rounded, larger pores); and from M. nitida due to its closely located gastropores (vs. few gastropores per cm2).
Distribution. Millepora laboreli has a very restricted distribution and is only known from the type locality, the Manuel Luiz Marine State Park. However, it was very abundant in this area.
Molecular systematics. Although, in general, the amount of data collected on the physical characters of Brazilian Millepora has proved adequate to differentiate between species, in some cases phenotypic plasticity may preclude positive identification and/or require substantial sample sizes. A complementary technique that can be used to validate identification involves analyses of molecular characters, and in recent years this has become popular in many studies, including those concerning marine invertebrates ( Solé-Cava and Thorpe 1991; Knowlton 1993). Although molecular techniques have been used in other cnidarians, such as hydroids ( Thorpe et al. 1992) and corals ( Stoddart 1983; Ayre and Wills 1988; Knowlton et al. 1992; Weil 1992; Garthwaite et al. 1994; Weil and Knowlton 1994), to date the only studies on the molecular systematics of calcified hydroids are by Manchenko et al. (1993), Amaral (1997), Amaral et al. (1997), Miller et al. (2004) and Lindner et al. (2008), the middle three being the only ones done with Brazilian species. Amaral et al. (1997) compared M. alcicornis and M. braziliensis by means of enzyme electrophoresis and confirmed what morphometric studies had already shown: there is no possibility of M. braziliensis being a synonym of M. alcicornis . Amaral (1997) also compared M. alcicornis and M. braziliensis to M. nitida , proving that these are three separate species. Molecular data should also be gathered in order to test the validity of Millepora laboreli .
Nematocysts. Among the species of Millepora (including M. alcicornis ) studied by Boschma (1949), only two types of nematocysts were observed, which Boschma (1949) called “larger nematocysts” and “smaller nematocysts”. For Brazil M. alcicornis, Amaral (1997) observed stenotele nematocysts with an average length of 44.6 Μm and holotrich nematocysts with a mean length and width of 24.7 Μm and 24.2 Μm, respectively. A single exploded nematocyst was observed: a holotrich, with a capsule measuring 26.2 Μm and a 275.1 Μm filament, totaling 301.3 Μm. Stenotele and holotrich nematocysts were also observed in M. braziliensis . The stenotele averaged 44.5 Μm in length, while the holotrich averaged 31 Μm in length and 27.8 Μm in width. Statistical analyses showed that the differences in the average length of these two species’ nematocysts were not significant. In M. nitida , a single holotrich nematocyst was observed, but was not measured.
Medusae. Amaral (1997) found medusae of M. alcicornis in the rainy season (June–August). Similarly, medusae of M. braziliensis were found in the beginning of the rainy season (March–June). The medusae of both species fit the description by Boschma (1956) “hydromedusae of reduced structure, for they lack velum, tentacles, and radial canals”. The M. alcicornis medusae averaged 17 μm in size, varying from 15 to 23 μm. Medusae from M. braziliensis were observed but not measured. Medusae from M. nitida and M. laboreli were not observed.
Ecology. Lewis (1989) noted the lack of information on the ecology of most species of Millepora , including those occurring in Brazil. Some information is available with respect to general characteristics such as preferred depth distributions and general habitats and their associated micro- and macrofauna and flora. Most of these observations are fairly recent, but compare favorably with earlier notes on Millepora from other regions of the world.
According to Lewis (1989), the genus Millepora occurs in depths from less than 1 m to about 40 m, but Amaral (1997) cited M. alcicornis occurring from 0 to 50 m. Likewise, to date there have been no comprehensive studies on this topic for Brazilian species, yet some ecological data can be found in other kinds of studies. Laborel (1969) recorded the following depth ranges for the Milleporidae of Brazil: 0–15 m for M. alcicornis , 1–5 m for M. braziliensis , and 0–5 m for M. nitida . More recently, Amaral et al. (2002) enlarged two of these species bathymetric distribution for Brazil: M. alcicornis was found up to 25 m deep and M. braziliensis was found at 30 m—both at the Manuel Luiz Coral Banks. Further, the bathymetric distribution of Millepora laboreli n. sp., endemic to the Manuel Luiz area, was recorded, with tall, unusually shaped colonies observed at depths of around 30 m. Although Laborel (1969) appointed specific reef zones for the occurrence of the different species of Brazilian Millepora , such as the reef front for M. braziliensis , these species have been observed in most reef regions and in other areas with coral fauna.
Amaral (1997) also observed some species of microflora associated with M. alcicornis and M. braziliensis ( Table 3 View TABLE 3 ). Special attention was paid to zooxanthellae in M. alcicornis , M. braziliensis , and M. nitida , in which all the phases of the life cycle of Symbiodinium sp. (according to Freudenthal 1962) were observed. There was a high relative density of these zooxanthellae and M. alcicornis had the highest density of the three species: the mean densities were 14.4 x 10 5 cells/cm2 for M. alcicornis , 4.5 x 10 5 cells/cm2 for M. braziliensis and 3.3 x 10 5 cells/cm2 for M. nitida . Statistical analyses showed that these differences were significant, although the low value for M. nitida might have been due to the fact that the studied specimens had been frozen, unlike the two other species, which were alive. In a study of the scleractinian coral Montastraea cavernosa from Mexico, Carricart-Ganivet and Beltrán-Torres (1993) found that the density was 1.99 x 10 5 cells/ cm2.
The diameters of the zooxanthellae were also examined by Amaral (1997). In M. alcicornis they varied from 7.9 to 26.2 Μm (12.5 Μm on average); in M. braziliensis the diameter ranged from 7.9 to 18.3 Μm (14.7 Μm on average); and in M. nitida the variation was from 10.5 to 18.3 Μm (15.1 Μm on average). The differences among the zooxanthellae diameters of the three species were found to be statistically significant. In addition, when compared to scleractinian corals the diameter of these zooxanthellae can be considered large (William Fitt, pers. comm.). For example, Amaral and Costa (1998) compared the zooxanthellae of M. alcicornis and M. braziliensis to that of the scleractinian corals Favia gravida and Siderastrea stellata and found that zooxanthellae of the latter had smaller mean diameters.
Few other aspects of the ecology or life histories of Brazilian Millepora have been documented; a bleaching event was observed for calcified hydroids in Brazil during a survey done in 1998 in the Manuel Luiz Coral Banks ( Amaral et al. 2006, 2007). All of the observed Milleporidae ( M. alcicornis , 25 m; M. braziliensis , 30 m; M. laboreli , 30 m) were bleached or dead and covered by algae and/or other organisms; only S. roseus was unaffected. According to the authors, the Millepora bleaching coincided with that of co-occurring scleractinians observed, apparently due to the same factors – an increase in the average sea surface temperature of at least 1°C higher than the previous year. Future studies should point to the impact of these bleaching events and overall climate change on the Brazilian hydrocoral species.
TABLE 2. Means (± SE) [number and range of measurements pooled across specimens] of various skeletal characters of the four Millepora species that occur in Brazil, according to Amaral (1997). (-) = the characteristic was not recorded, Dia = diameter, No = number. All measurements are in mm.
| Characteristic | M. alcicornis | M. braziliensis |
|---|---|---|
| (34 colonies) | (45 colonies) | |
| Height of colony | 138.20 (11.31) [23; 53.7–306.0] | 109.00 (7.04) [41; 36.5–217.8] |
| Transverse diameter | 103.60 (8.82) [22; 35.0–188.0] | 102.10 (5.73) [35; 50.0–190.0] |
| No principal branches | 1.90 (0.22) [23; 1–5] | 3.20 (0.62) [16; 1–7] |
| Dia of principal branch | 22.85 (1.913) [26] | 20.20 (2.63) [50] |
| Dia of terminal branch | 6.65 (0.34) [491] | 24.60 (1.56) [144] |
| No of ampullae | 11.08 (2.08) [23; 1–44] | 6.50 (1.41) [23; 1–27] |
| Dia of ampullae | 0.41 (0.02) [171] | 0.53 (0.01) [136] |
| No of cyclosystems | 1.10 (0.037) [32; 1.0–1.5] | 2.00 (0.10) [38; 1.0–3.5] |
| No of epibionts | 16.80 (5.18) [12; 1–50] | 5.40 (2.07) [14; 1 – 30] |
| Dactylopores per gastropore | 5.20 (0.13) [8; 5.0–5.5] | 7.75 (0.72) [4; 5.0–7.5] |
| Dia of dactylopores | 0.14 (0.001) [872] | 0.10 (0.001) [1130] |
| Dia of gastropores | 0.29 (0.002) [827] | 0.25 (0.001) [1152] |
| No of gastropores per cm2 | 29.25 (3.17) [35; 4–88] | 18.15 (1.41) [39; 4–48] |
| continued. |
TABLE 3. Associated fauna and flora to three Millepora species that occur in Brazil, according to Amaral (1997), excluding zooxanthellae.
| M. alcicornis (34 colonies) | M. braziliensis (45 colonies) | M. nitida (13 colonies) | |
|---|---|---|---|
| Associated microfauna | Ciliated protozoa, including Euplotes sp.; flagellated protozoa ( Euglena sp.); Cyprinidae ostracods | Ciliated protozoa, (including Euplotes sp.), flagellated proto- zoa ( Euglena sp.), Cyprinidae ostracods, and Tunicata Oiko- pleura. | |
| Associated macrofauna | Unidentified species from Phylum Sar- comastigophora ( Homotrema sp.), Phy- lum Porifera (occurring in the dead parts of some colonies), Phylum Cnidaria ( Favia gravida Verrill, 1868 and Sid- erastrea stellata Verrill, 1868 ), Phylum Mollusca ( Lithophaga nigra (Orbingny) and Noetia bisulcata (Lamarck)) , Phy- lum Annelida (families Nereidae , Sabel- lidae, Serpulidae , and Syllidae ), Phylum Crustacea ( Megabalanus stultus (Dar- win), Eriphia gonogra (Fabricius) , Pachycheles sp., and Family Alpheidae ), Phylum Bryozoa, and Phylum Echino- dermata ( Ophiothrix angulata Say ). | Phylum Cnidaria ( Agaricia agaricites (Linnaeus) ; Favia gravida Verrill ; and Sideras- trea stellata Verrill), Phylum Mollusca ( Lithophaga nigra (Orbingny) ; Noetia bisulcata (Lamarck) , Petaloconchus macrophragma Carpenter , and P. erectus (Dall)) , Phylum Annelida (families Serpulidae , Sabellidae , and Syllidae ), Phy- lum Crustacea ( Megabalanus stultus (Darwin)) , and Phylum Bryozoa. | Phylum Cnidaria ( Agaricia agaricites (Linnaeus)) , Phylum Annelida (only tubes were observed and identifi- cation was not possible), Phylum Crustacea (uniden- tified Cirripedia), and Phy- lum Bryozoa. |
| Associated microflora | The diatoms Climacosphenia moniligera (Ehrenberg) , Cylindrotheca closterium (Ehrenberg) , Mastogloia sp., Navicula sp., Nitzschia sp., and Cocconeis scutel- lum (Ehrenberg), and the Crysophyta algae Symbella sp. | The diatoms Climacosphenia moniligera (Ehrenberg) , Cylin- drotheca closterium (Ehren- berg), Mastogloia sp., Navicula sp., Nitzschia sp., and Amphora sp. | |
| Other | Cyanobacteria: Croococales sp., Spir- ulina sp., and Lyngbya sp.; unidentified bacteria. | Cyanobacteria: Croococales sp., Spirulina sp., and Lyngbya sp.; unidentified bacteria. |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |