Pseudolycoriella macrotegmenta, in Mohrig & Jaschhof, 1999
|
publication ID |
https://doi.org/10.11646/zootaxa.4707.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:3C00FB35-708D-4FF0-94C2-B15BD2A1F37A |
|
persistent identifier |
https://treatment.plazi.org/id/03D43F59-3643-FFDB-FF32-F8D031FD3D78 |
|
treatment provided by |
Plazi |
|
scientific name |
Pseudolycoriella macrotegmenta |
| status |
|
Pseudolycoriella macrotegmenta View in CoL complex
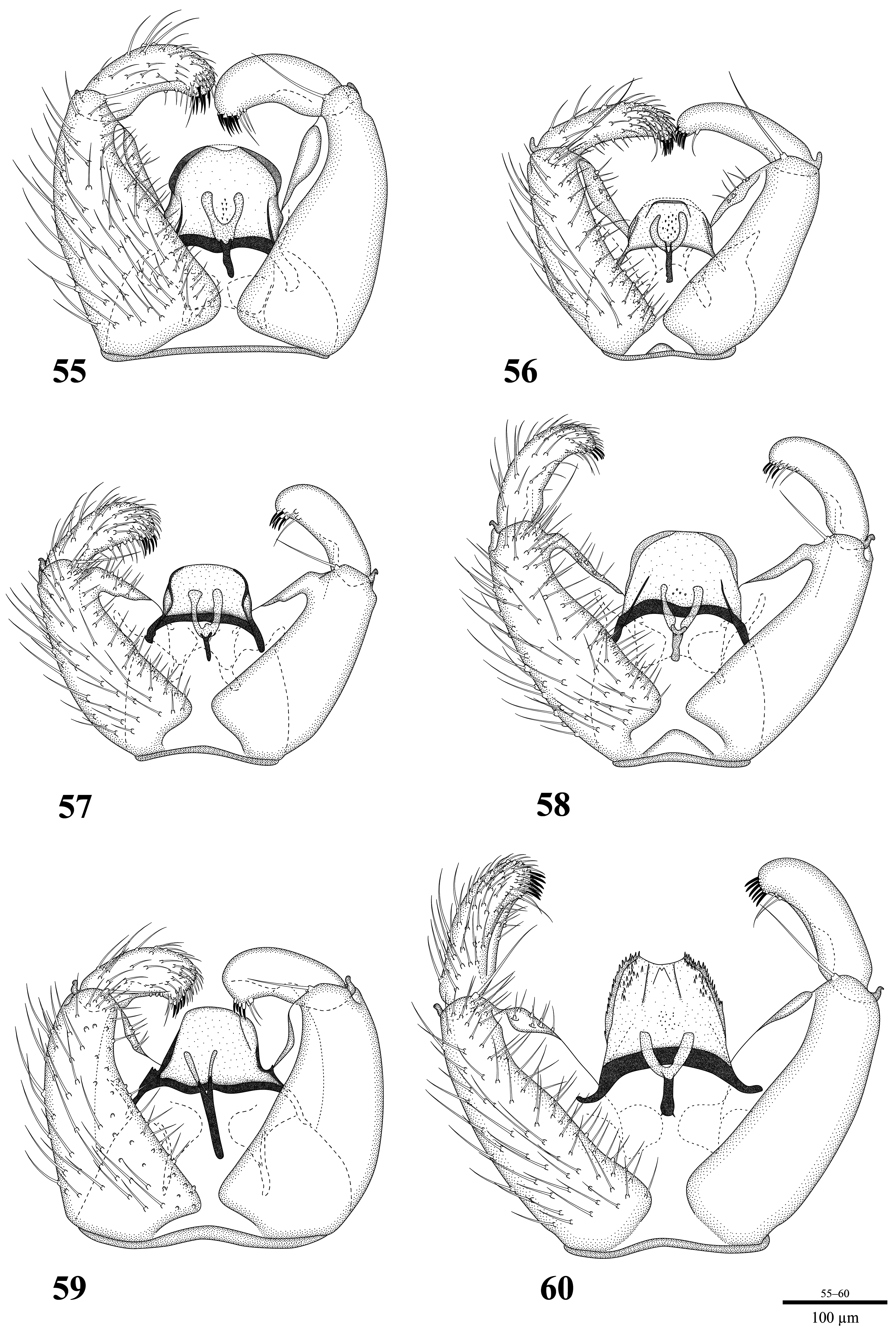
Thirteen of the species examined during this study have a very similar hypopygia structure. The number of gonostylar spines, ranging from four to ten, is greater than in most other Pseudolycoriella species. The length of those spines is also reduced. Consequently, the spines are very difficult to distinguish from the surrounding setae at the apex of the gonostylus. The apical whip-lash hair is also mostly reduced in length, and in several species two or even three hairs are present. The tegmen possesses a variety of unique characters. Some species bear teeth on the lateral margin of the tegmen ( lt in Fig. 52 View FIGURES 48–53 ). In the most derived state these teeth cover the whole apicolateral part of the tegmen ( Fig. 60 View FIGURES 55–60 ). On the dorsal side of the tegmen of some species there are creases arising from the outer base and converging towards the apex. These dorsal structures ( ds in Fig. 52 View FIGURES 48–53 ) can vary in length and number. The phylogeny of the Psl. macrotegmenta complex is shown in Figure 61 View FIGURE 61 .
Discussion. An assignment of this assemblage of species to one of the existing species groups of Pseudolycoriella was not possible, as already mentioned by Köhler & Mohrig (2016) for Psl. frederickedwardsi and Psl. tonnoiri . With the bulging prefrons and clypeus, and the ground plan of the tibial organ this species complex is undoubtedly part of the genus Pseudolycoriella . The increase in the number of gonostylar spines and the reduction of the length and the multiplication of the whip-lash hair can be considered to be morphological evidence for the monophyly of the Psl. macrotegmenta complex. The result of the molecular analysis also strongly supports the monophyly ( Fig. 61 View FIGURE 61 ). With the numerous gonostylar spines and the reduction of the length of the whip-lash hair the Psl. macrotegmenta complex resembles the Psl. quadrispinosa group from Papua New Guinea (Mohrig 2013). However, in the Psl. quadrispinosa group the gonostylar spines are easily distinguishable from the surrounding setae and the whiplash hair is lost, except in Psl. bitorquia Mohrig where a small whip-lash hair persists. In the Psl. macrotegmenta complex the spines are not as easy to distinguish from the surrounding setae and the whip-lash hair is never completely reduced, only shortened. Further, several species of the Psl. macrotegmenta complex possess two or three whip-lash hairs. Thus, the gonostylar spines and the whip-lash hair(s) do not necessarily share the same taxonomic value and it is likely that their appearance is a result of convergence. Another similar species is Psl. curvimedia Mohrig & Rulik—a species recorded from the Dominican Republic. It also has a similar pattern of spines on the gonostylus. However, there are other characters that differ significantly from those in the Psl. macrotegmenta complex and therefore do not support integration into the Psl. macrotegmenta complex. The flagellomeres of Psl. curvimedia have a rather smooth surface ( Mohrig et al. 2004), while those of the species of Psl. macrotegmenta complex are rough with deep pits. Also, the wing venation of Psl. curvimedia is very aberrant in comparison to representatives of the Psl. macrotegmenta complex. Pseudolycoriella curvimedia shows a very short R 1 that only comprises a fourth of R, a very unusual M-fork, and a nearly rectangularly bent CuA 2. Furthermore, the gonocoxites of Psl. curvimedia are fused ventrally, while they are widely separated in the Psl. macrotegmenta complex. Another similar species is the Australian Psl. rubroalata Mohrig, Kauschke & Broadley, which also bears eight to ten short spines ( Mohrig et al. 2018), but shows none of the characters on the tegmen that occur in the New Zealand Psl. macrotegmenta complex. However, without an accurate examination, preferably incorporating genetic analyses, a well-founded conclusion about the relationship of the Psl. quadrispinosa group, Psl. curvimedia , and Psl. rubroalata to the species of the Psl. macrotegmenta complex is not possible. Until such an examination, it remains unclear whether these species belong to the Psl. macrotegmenta complex or whether the similar patterns of gonostylar spines are a result of convergence. Nevertheless, it can be stated that surely these species do not belong to the New Zealand crown group of the Psl. macrotegmenta complex. The combination of an increased number of reduced (i.e. thinner) spines and flagellomeres with a pitted surface can be held to be a synapomorphy for the New Zealand members of this complex. Apart from the morphological considerations noted above, the very small genetic distances between the New Zealand representatives of this complex indicate a more recent radiation of these species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |