Isabella tanoa, Ekins, Merrick, Erpenbeck, Dirk, Wörheide, Gert & Hooper, John N. A., 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4136.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:DBD1B993-6DF8-4E23-BD27-FA5E0036EE0B |
|
DOI |
https://doi.org/10.5281/zenodo.5695901 |
|
persistent identifier |
https://treatment.plazi.org/id/FBAB24FD-3903-4475-9443-49DE8E8BF89B |
|
taxon LSID |
lsid:zoobank.org:act:FBAB24FD-3903-4475-9443-49DE8E8BF89B |
|
treatment provided by |
Plazi |
|
scientific name |
Isabella tanoa |
| status |
sp. nov. |
Isabella tanoa View in CoL n. sp.
( Figs 6–12 View FIGURE 6 View FIGURE 7 View FIGURE 8 , Table 1 View TABLE 1 )
Material examined. Holotype - MNHN DCL 4111 (fragment of holotype QM G318737) Antigonia seamount, Norfolk Ridge, 23.367 S, 168.034 E, 180–250 m, RV Alis, 26/06/2001, Waren dredge, coll. Bertrand Richer de Forges, Tin-Yam Chan, Bernard Cohen, Enrique MacPherson, Marie-Catherine Boisselier, Pierre Lozouet. Paratypes - QM G318765, G335130 Jumeau West seamount, Norfolk Ridge 23.669 S 168.009 E, 237–250 m, RV Alis, 21/06/2001, Waren dredge, coll. Bertrand Richer de Forges, Tin-Yam Chan, Bernard Cohen, Enrique Mac- Pherson, Marie-Catherine Boisselier, Pierre Lozouet. G318803 Blanc Nouveau 2 seamount, Norfolk Ridge, 23.302 S, 168.247E, 185–207m, RV Alis, 27/06/2001, Waren dredge, coll. Bertrand Richer de Forges, Tin-Yam Chan, Bernard Cohen, Enrique MacPherson, Marie-Catherine Boisselier, Pierre Lozouet.
Description. Growth Form. Massive shallow cup like sponge, with a broad base and an even margin. Dimensions of the holotype are 59 mm long, 49 mm wide and 31 mm high. The other specimens range from 50–82 mm in diameter and 16–42 mm high. As one of the paratypes (G335130) appears to be a triangular sector from a circular dish, we suspect this species could grow significantly larger than recorded here ( Fig. 6 View FIGURE 6 ).
Colour. Wine dark purple on deck and wine dark purple/brown in ethanol ( Fig. 6 View FIGURE 6 ).
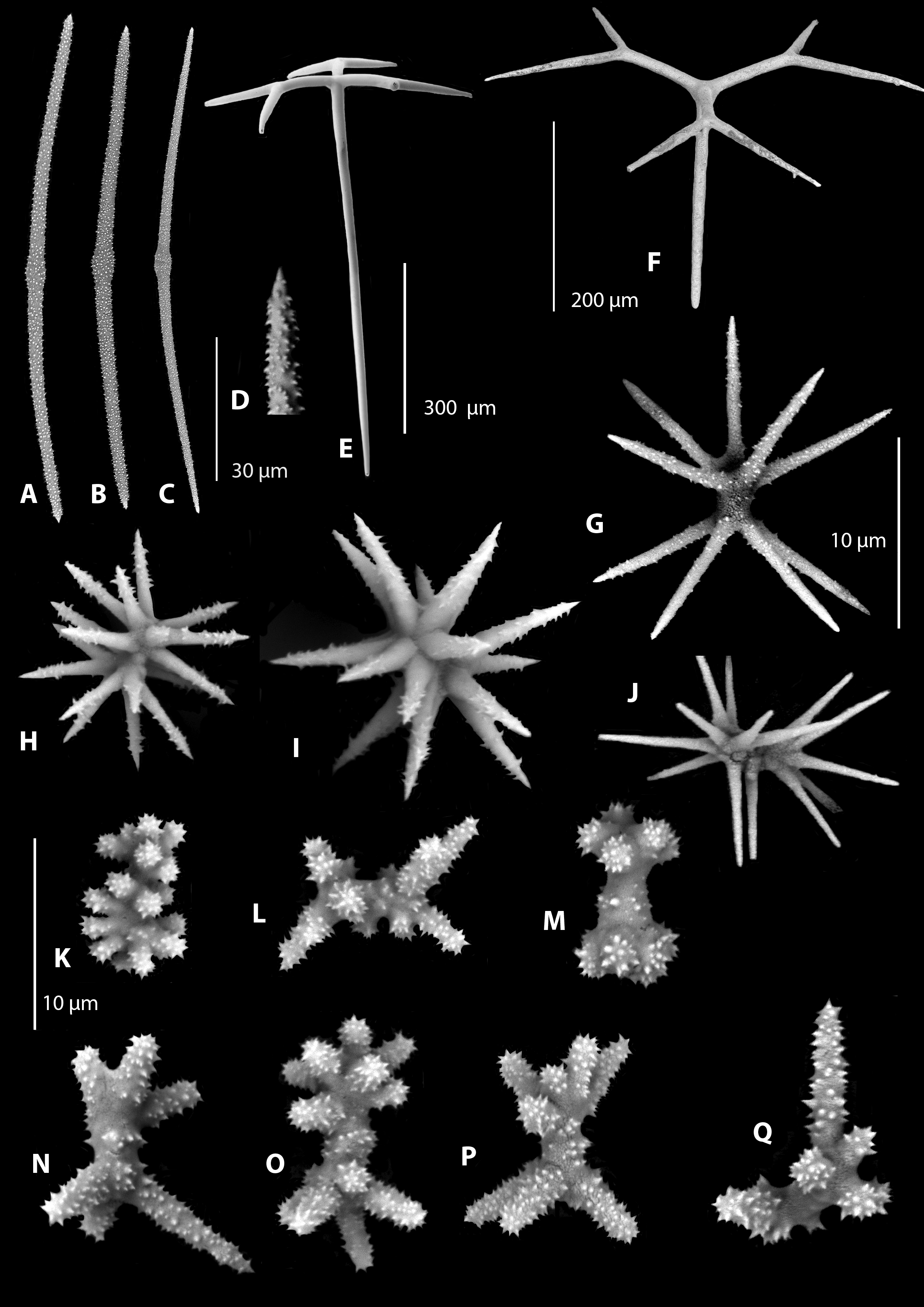
Oscules. There are numerous microscopic exhalant oscules 0.05–0.2mm in diameter ( Fig. 6 View FIGURE 6 ), also seen to be minute under scanning electron microscopy ( Fig. 7 View FIGURE 7 ).
Texture. Hard, stony but compressible and slightly hispid with a brittle compressible ectosomal crust.
Surface ornamentation. Slightly hispid caused by oxeas protruding several millimetres above the surface every couple of millimetres ( Fig. 7 View FIGURE 7 ).
Ectosomal skeleton. There is a thin (1mm) ectosomal crust of microscleres with smooth dichotriaenes perpendicular to the surface. The microscleres consist of abundant asymmetrical microspinose spiraster streptasters with short thick arms, and microxeas. The microxeas consist of thin microspinose centrotylote microxeas and microspinose microxeas. There are also brushes of long smooth oxeas, which originate in the choanosome and punctuate the surface by several millimetres. There is occasionally detritus embedded in the ectosome.
Choanosomal skeleton. The skeleton in the subectosomal region of the choanosome consists of a 3D matrix of regular and slightly curved dicranoclone desmas with some tubercles. Further into the choanosome the desmas change from long thin graceful dicranoclones into heavier interlocked dicranoclones with multiple zygosis. Choanosomal microscleres consist of microspinose spiraster streptasters with slender arms and occasional centrotylote oxeas. There are bundles of smooth very long curved oxeas that extend through the ectosome to the surface.
Megascleres. There are two main smooth ectosomal dichotriaenes , one has delicate long shafted dichotriaenes with acerate rhabdome tips. There are also and slightly more commonly encountered delicate short-shafted dichotriaenes with smooth rounded rhabdome tips. Subectosomal choanosomal dicranoclone desmas are smooth and slender with small terminal tubercules. Dicranoclone desmas deep within the choanosome are thicker, interlocking, with more numerous tubercles with saddle zygoses. Oxeas are very long thin and sharp originating in the choanosome and extending in bundles out to the exterior of the sponge (see Fig. 7 View FIGURE 7 ).
Microscleres. Centrotylote microacanthoxeas are very common. The microscleres consist of abundant symmetrical microspinose spirasters with long thin arms and asymmetrical microspinose streptasters with short thick arms.
DNA signature sequence. CO1 sequences are deposited in NCBI Genbank (Accession number KX267961 View Materials – KX267963 View Materials ) and the Sponge Barcoding Database (Accession number 1643–1645).
Etymology. Tanoa is the name of the mixing bowl used in the Yangona drinking ceremony in the Pacific Islands. The morphology of the sponge shares the basic bowl shape of the Tanoa .
Isabella tanoa sp nov QM G Isabella tanoa sp nov QM G Isabella tanoa sp nov QM G Isabella harborbranchi KM
Isabella harborbranchi KM Isabella harborbranchi KM
Isabella mirabilis genotype F QM G
Isabella mirabilis genotype F QM G
Isabella mirabilis genotype F QM G
Isabella mirabilis genotype F QM G
Isabella mirabilis genotype F QM G
Isabella mirabilis genotype F QM G
Isabella mirabilis genotype F QM G
Isabella mirabilis genotype F QM G
Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype C QM G Isabella mirabilis genotype D QM G Isabella mirabilis genotype D QM G Isabella mirabilis genotype E QM G Isabella mirabilis genotype E QM G Isabella mirabilis genotype E QM G Isabella mirabilis genotype E QM G Isabella mirabilis genotype E QM G Isabella mirabilis genotype E QM G
Neoaulaxinia zingiberadix QM G
Neoaulaxinia zingiberadix QM G
Neoaulaxinia zingiberadix QM G
Neoaulaxinia zingiberadix QM G
Neoaulaxinia zingiberadix QM G
Neoaulaxinia zingiberadix QM G
Neoaulaxinia zingiberadix QM G
Neoaulaxinia zingiberadix QM G
12 I. tanoa I.mirabilis 10 genotype F I. mirabilis 8 genotype C I. mirabilis genotype D
Spicule width
(µm) 6 I. mirabilis genotype E
4 2 0
0 20 40 60 80 100 120 140 160
Spicule Length (µm)
35 I. tanoa
30 I. mirabilis genotype F
25 I. mirabilis genotype C
20 I. mirabilis genotype D Spicule Width I. mirabilis genotype E (µm) 15
10
5
0
0 5 10 15 20 25 30 35
Spicule Length (µm)
Remarks. Morphological distinction of Isabella spp. Isabella tanoa’s overall morphology resembles a Tanoa or Yangona bowl or mortar. The two paratypes resemble shallow bowls, and have similar colour and texture to both I. mirabilis and I. harborbranchi , whereas the holotype of I. tanoa lacks the characteristic warts that give I. mirabilis its warty appearance.
Isabella tanoa differs from both I. mirabilis and I. harborbranchi in not having any smooth microxeas. Although microxeas are not specifically mentioned in the description of Carvalho et al. (2015), they include in their table oxeas whose smallest size is 100 µm long. The centrotylote microacanthoxeas are listed as rare in I. harborbranchi but abundant in I. tanoa and I. mirabilis .
Isabella mirabilis also has smooth centrotylote microxeas. The microspined microxeas in I. tanoa are generally more slender than those in I. mirabilis and I. harborbranchi . I. tanoa lacks the fusiform microacanthoxeas found in I. mirabilis and I. harborbranchi and the long thin blunt oxeas found in I. harborbranchi .
Isabella tanoa and I. mirabilis both have extremely long and slightly curved oxeas. However they are longer and thicker in I. tanoa . Both I. tanoa and I. mirabilis species have long oxeas, which pierce the surface in brushes, extending several millimetres above the sponge surface. Isabella tanoa does not have either of the shorter oxeas found in I. harborbranchi .
Isabella tanoa lacks the long and short-shafted triaenes present in I. mirabilis and I. harborbranchi . Isabella tanoa has short-shafted dichotriaenes with rounded rhabdome tips, that are also present in I. mirabilis , however the dichotriaenes in I. tanoa are delicate and slender and only half the width of the stouter I. mirabilis dichotriaenes . All three species have similar large spirasters with long thin arms. Isabella mirabilis contain thick streptasters ‘Jacks’ that do not occur in either I. tanoa or I. harborbranchi . However, only I. tanoa and I. harborbranchi have the long spirasters with short thick thorny arms.
Isabella tanoa has a combination of both graceful desmas in the subectosome region and the interlocking desmas as in I. mirabilis in the choanosomal skeleton. Both I. tanoa and I. mirabilis have an ectosomal skeleton which is easily differentiated and separable from the choanosomal skeleton, unlike in the skeleton of I. harborbranchi .
Molecular distinction of I. tanoa sp. nov. The final data set consisted of 56 taxa and resulted in a dataset of 563 characters of which eleven (= 2%) were variable among the Isabella spp (see Table 2). In I. mirabilis the CO1 sequences differentiated specimens into four distinct genotypic groups labelled here as genotype C to F (see Ekins et al. (2015) with 0.2–0.5% (uncorrected p-) difference. CO1 sequences were identical for all the specimens of I. tanoa , but differed from sequences of I. mirabilis by 0.9–1.1% and I. harborbranchi by 1.1% (see Table 2). Maximum likelihood reconstructs the three species as highly supported monophyletic with a sister group relationship between I. harborbranchi and I. tanoa .
Taxon\Position* 318 406 408 410 411 420 426 438 456 618 654 I. mirabilis C A G A A A T A G T G G I. mirabilis D A G A A A T A G T T G I. mirabilis E A G A A A T T G T G G I. mirabilis F A G A A A G T G T G G I. tanoa sp. nov. A C T C C G T G T G T I. harborbranchi G A T C A G T A C G G
* referring to A. queenslandica CO1 (NCBI Genbank NC 008944)
Comparative morphometric analysis and variability. The length and width measurements of the spirasters of I. mirabilis and I. tanoa ( Fig. 11), show a large overlap with I. tanoa at the smaller end of the scale. However, the differences between the two species, I. mirabilis and I. tanoa , are highly significant (P <0.001) across all of the genotypes for both measurements. There are also some slight variations within and between some genotypes of I. mirabilis . Dichotriaenes are extremely variable in shape and size in I. mirabilis ( Fig. 12), with variation between individuals being more pronounced than the variation between the genotypes. However, the differences between the two species are very obvious despite these shape and size variations, as shown in Figure 12, where the major differences are in the thickness of the rhabdome and dichotriaene arms (P <0.001). There are also significant differences in the diameter of the cladome (P <0.05) and length of the rhabdome (P <0.001) between I. tanoa and all the genotypes of I. mirabilis . The delicate dichotriaenes of I. tanoa dichotriaenes also separate readily into short and long shafted dichotriaenes as seen in Figure 12.
80 I. mirabilis genotype D
70 I. mirabilis genotype E) µm 60
width 50 I. tanoa long shafted Rhabdome 40 30 I dichotriaenes dichotriaenes. tanoa short shafted 20
10
0
0 200 400 600 800 1000 1200 1400
Rhabdome length (µm)
12 I. mirabilis genotype C
10 I. mirabilis genotype D
Spicule Width 6
(µm)
4 2 0
0 50 100 150
Spicule Length (µm)
Some significant differences were found in measurements of lengths and widths of the centrotylote microacanthoxeas for I. mirabilis and I. tanoa (as shown in Figure 10). However, the differences between the two species I. mirabilis and I. tanoa , are unique in that they are highly significant across all of the genotypes (P <0.001). Measurements of centrotylote microacanthoxeas for individual specimens of I. mirabilis show that significant differences were found within the four genotypes of this species (see Table 2), overshadowing any significant differences between the genotypes, such that morphometric differences between specimens were masked. Some specimens also had a larger number of centrotylote microacanthoxeas than non-centrotylote microacanthoxeas, some had triaenes, and some showed differences in the shape of their dichotriaenes , but these trends cut across the different genotypes. The variation within and between the specimens highlights the variability of these spicules within this species.
10
9
8
7 I. mirabilis genotype D
6 I. mirabilis genotype E
Spicule Width 5
4
3
2
1
0
0 5 0 1 0 0 150 200 250 300
Spicule Length (µm)
10
9
8 I. mirabilis genotype D
7 I. mirabilis genotype E
6 I. mirabilis genotype F
Spicule Width 5
(µm)
4
3
2
1
0
0 100 200 300 400
Spicule Length (µm)
25
20 I. mirabilis genotype D
15 I. mirabilis genotype F
Spicule Width (µm)
10
5 0
0 5 10 15 20 25
Spicule Length (µm)
Comparisons of lengths and widths of the non-centrotylote microacanthoxeas, smooth centrotylote microxeas, smooth non-centrotylote microxeas and ‘Jack’ streptasters spicule types between and within the four genotypes of I. mirabilis indicate that they are similar to each other ( Figs 13–16). Whilst significant differences do exist between some combinations of genotypes, there are never any consistent significant differences between any two genotypes (see Appendix/ Supplementary Material). Moreover there are significant differences between individuals within each genotype for these spicule types, indicating that measurements within this species are insufficient to justify the separation into four separate species.
The absence of any triaene in genotype D of I. mirabilis is the most obvious difference between the four genotypes of this species. However, there are only two specimens of this genotype and the reality is that triaenes were not observed in all specimens of the other genotypes either (including the holotype) (see Table 2), and as such this character is interpreted as a secondary loss within specimens of this species.
| MNHN |
Museum National d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Astrophorina |
|
Family |
|
|
Genus |