Stegonotus aplini, O’Shea & Richards, 2021
|
publication ID |
https://doi.org/10.11646/zootaxa.4926.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:49BEE762-97B0-4CD8-B942-7241EB220138 |
|
DOI |
https://doi.org/10.5281/zenodo.4531888 |
|
persistent identifier |
https://treatment.plazi.org/id/03D687F7-FFDB-464C-B9F8-572AAB9E5F46 |
|
treatment provided by |
Plazi |
|
scientific name |
Stegonotus aplini |
| status |
sp. nov. |
Stegonotus aplini View in CoL sp. nov.
urn:lsid:zoobank.org:pub: ( urn:lsid:zoobank.org:act:)
Purari Groundsnake
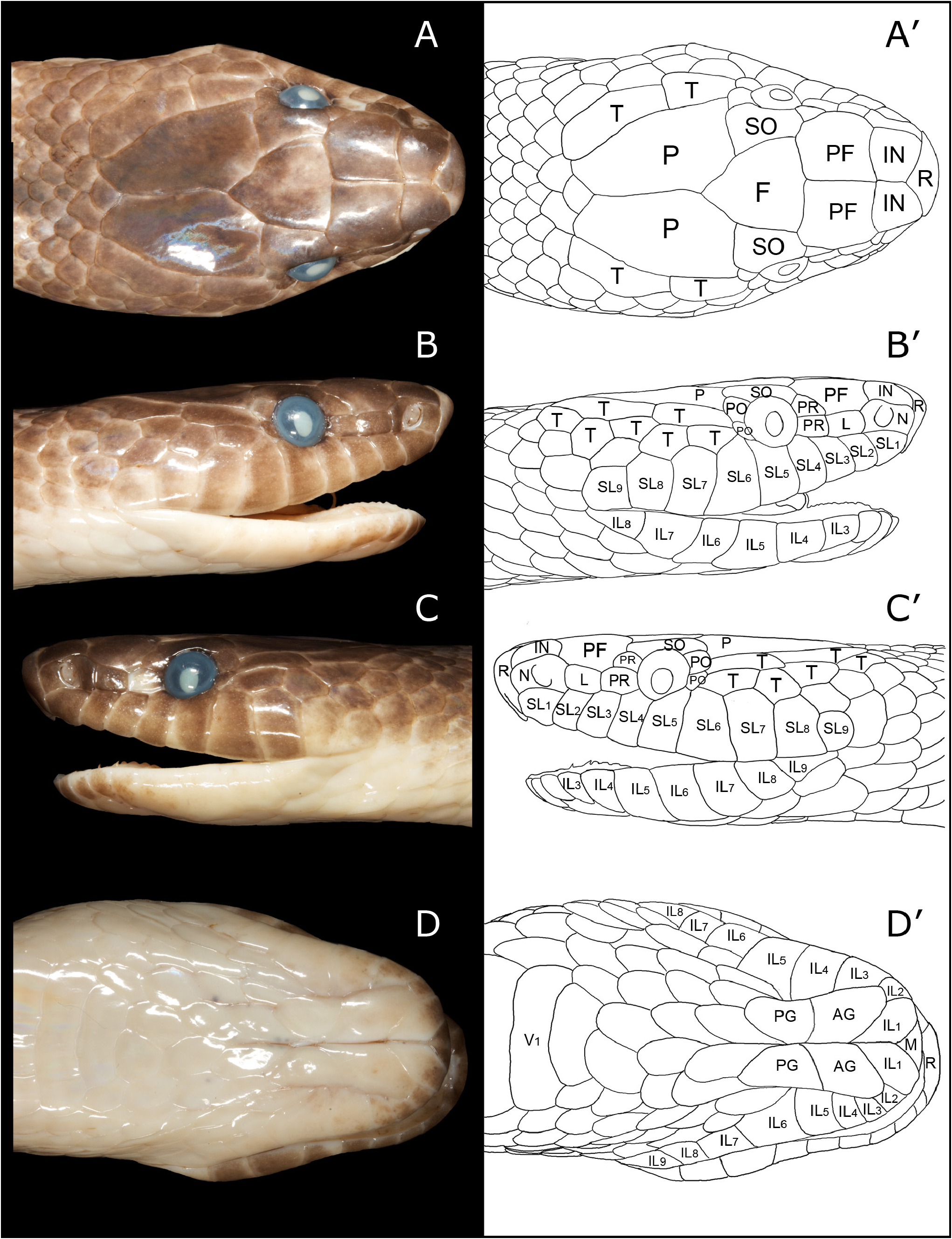
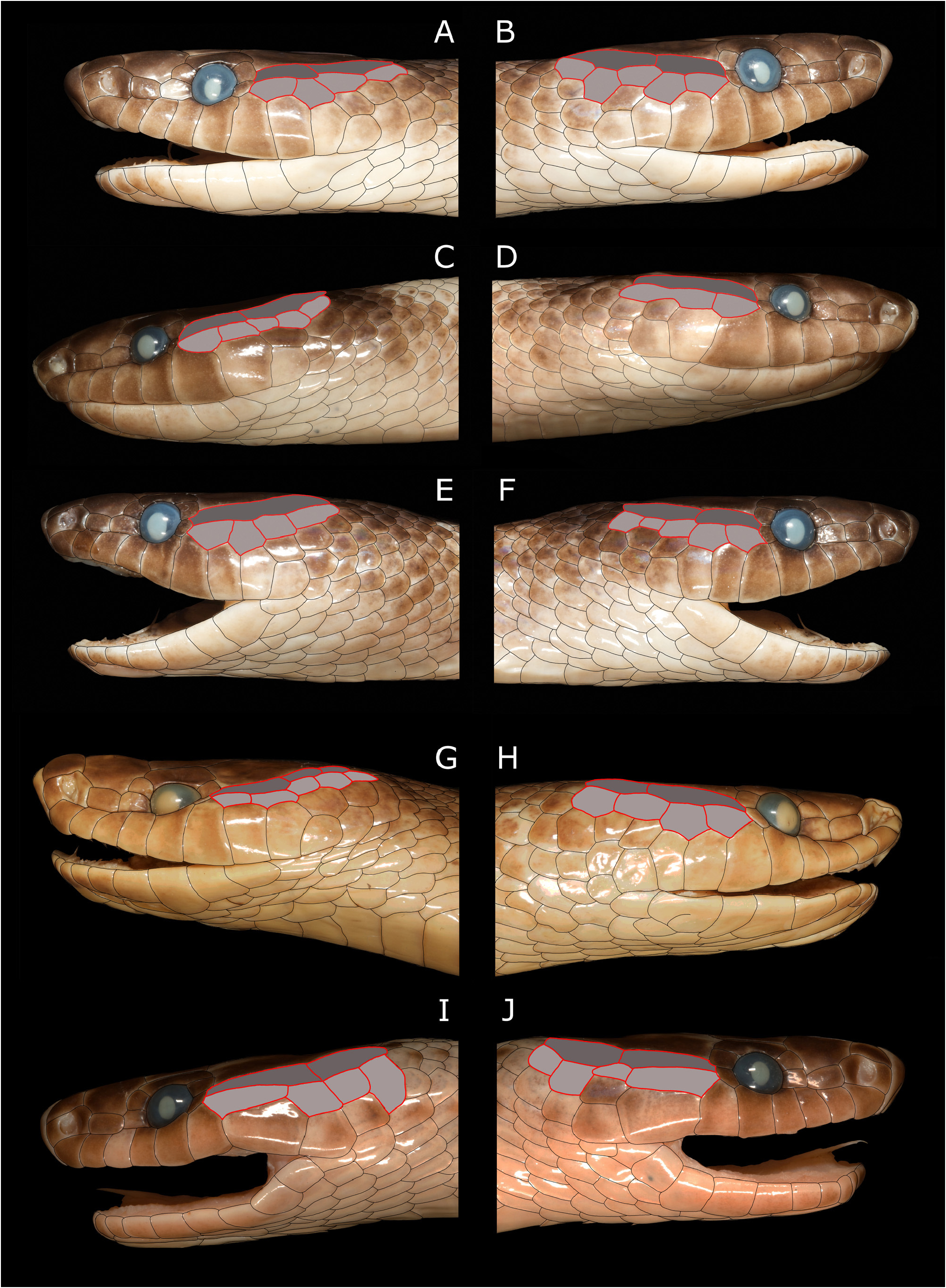
Figs. 1 View FIGURE 1 , 2 View FIGURE 2 , 3 View FIGURE 3 A–H, 4A–D
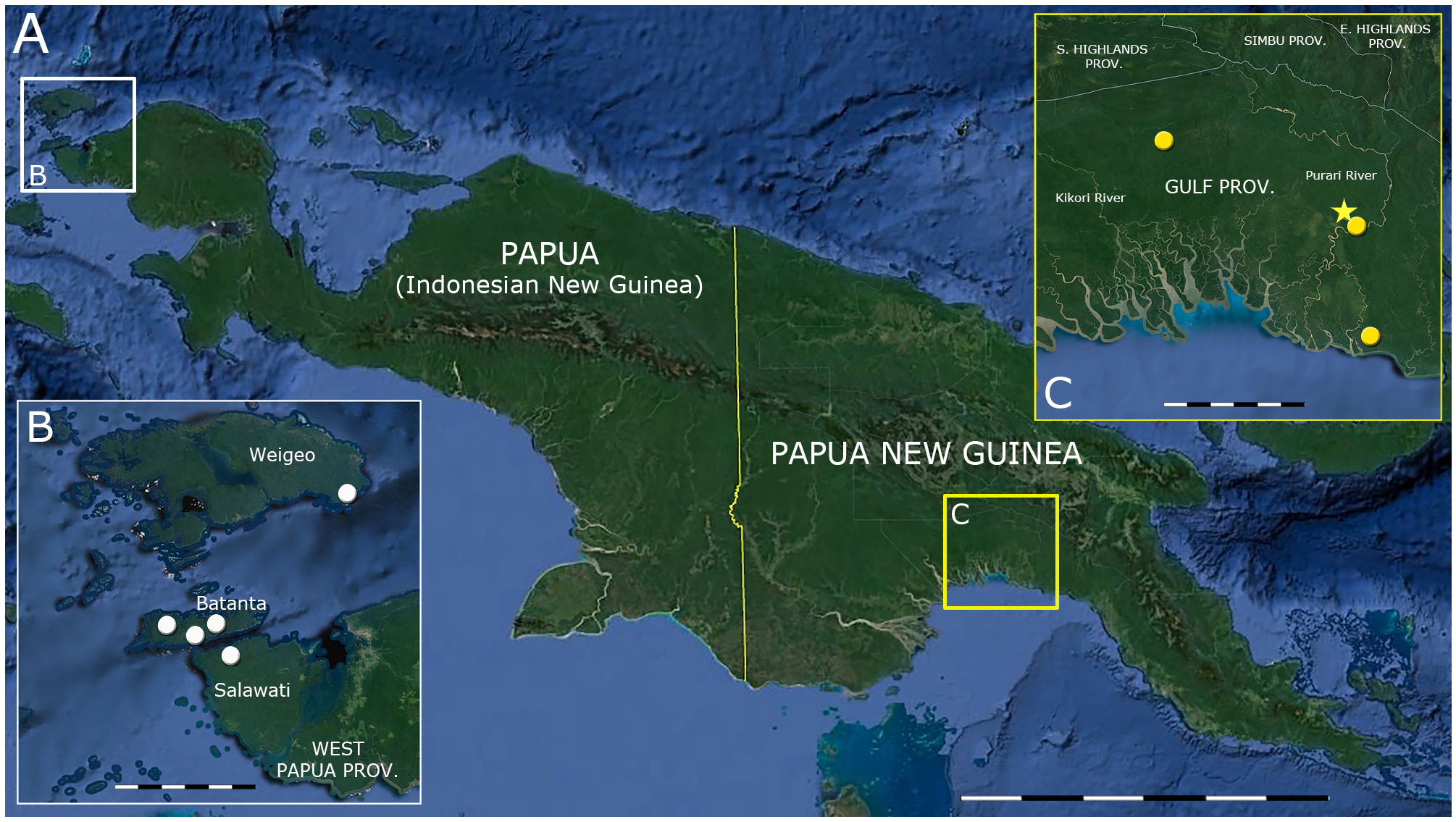
Holotype. SAMA R71442 (field number SJR 15138), an adult male from ca. 12 km NW of Orloli, Purari River basin, Gulf Province, PNG ( 7.3126°S, 145.1378°E, elevation ca. 20 m), collected by Ken Aplin on 22 June 2016.
Paratypes. PNGNM R25322 (field number SJR 15115), an adult male from ca. 6 km N of Orloli, Purari River basin, Gulf Province, PNG ( 7.3510°S, 145.1900°E, elevation ca. 30 m), collected by Ken Aplin on 11 February 2016; SAMA R71443 (field number SJR 15330), an adult male from ca. 1.6 km NW Muro Mission, Purari River basin, Gulf Province, PNG ( 7.7890°S, 145.2660°E, elevation ca. 5 m), collected by S. Richards and E. Nagombi on 12 July 2016; AMS R.13302, an adult male from the Kereru Range on the Abede River, Gulf Province, Papua New Guinea (ca. 7.0280°S, 144.4226°E, elevation uncertain) by geologist J. P. de Verteuil, probably in 1947 (collection date unavailable but catalogue entry dated 6 January 1948).
Etymology. The species epithet is a patronym to honour Dr. Ken Aplin (1958–2019), in recognition of his immense contributions to New Guinean herpetology, and in gratitude for his friendship and selfless collaboration with the junior author over many years. Ken’s many experiences in Melanesia have added significantly to our knowledge of the region’s vertebrate fauna, both living and fossil, and his recent passing has created a void that will be hard to fill.
Diagnosis. A species of Stegonotus that can be diagnosed from all congeners by the following combination of characters: dorsum of body immaculate snow-white anteriorly, with dark speckling that manifests as occasional brown-tipped scales by midbody and increases in frequency and density posteriorly; tail entirely dark brown with light pigment confined to lowest dorsal scale rows; venter and subcaudal scales off-white; head dorsally dark brown, contrasting with white anterior of the body, brown pigment extending posteriorly for 6–20 scales; dorsal scales rows 17-19-15 (75%) or 19-19-15 (25%), ventral scales 229–239, subcaudal scales 83–95, all divided; SL usually nine (75%), occasionally eight (25%), with SL4–SL5 contacting the orbit; and IL nine (75%), or eight (25%) with IL1–IL4 or IL1–IL5 contacting the anterior genials.
Description of holotype. ( Figs. 1 View FIGURE 1 A–D, 2A–B). An adult male, SVL = 1087 mm, TL = 290 mm, TTL = 1377 mm; head distinct from neck, snout rounded in dorsal view, body arch-shaped in cross-section, tail moderately long (TL 21% of TTL). Dorsal scales in 17-19-15 rows; 229 ventrals, 89 paired subcaudals, and an entire cloacal plate; SC/(V+SC) ratio = 0.28. Rostral clearly visible when viewed from above, extending back to separate internasals for one-third their depth but not to a point level with the nostrils (shallow V condition; Kaiser et al., 2019). Internasals paired, in broad contact behind rostral. Prefrontals also in broad contact, twice as long and 1.3 times as wide as internasals, extending laterally onto head to contact preoculars, loreal and nasal. Frontal shield-shaped, as broad as long. Supraoculars much broader posteriorly than anteriorly. Parietals twice as long as wide. Nasal almost split by large naris, almost reaching first supralabial. Loreal rectangular, 1.5 times as long as high. Two preoculars, lower largest, nearly equal in length to loreal. Two postoculars, upper larger. Nine supralabials, SL4–SL 5 in contact with orbit, SL6–SL7 largest, SL5–SL6 contacting lower postocular. Two elongate temporals on left side ( Figs. 1 View FIGURE 1 C–C’, 3A), whose length when combined equals length of left parietal; upper anterior temporal contacting both postoculars, broadly bordered below by a pair of lower anterior temporals and the first of two lower posterior temporals; upper posterior temporal bordered below by two lower posterior temporals. Two elongate temporals on right side ( Figs. 1 View FIGURE 1 B–B’, 3B), whose length when combined equals length of right parietal; upper anterior temporal contacting both postoculars, broadly bordered below by a pair of lower anterior temporals and the first of three lower posterior temporals; upper posterior temporal bordered below by three lower posterior temporals. Infralabials nine on left side, eight on right side, with IL1–IL5 on left side and IL1–IL4 on right side in contact with the anterior genials, due to division of IL3 on the left side, and IL5–IL6 and IL4–IL5, respectively, contacting posterior genials. Mental relatively small, triangular, followed by enlarged paired IL1, which exhibit broad contact at midline. Anterior genials large and separated by mental groove, posterior genials smaller than anterior genials, also separated by the continuing mental groove. Three to four alternating chin scales separate genials from first gastrostege (ventral).
Variation. All three paratypes ( Figs. 2 View FIGURE 2 C–H) are adult males with the following measurements: PNGNM R25322 with 1090 mm SVL + 285 mm TL = 1375 mm TTL, TL = 20.7% of TTL; SAMA R71443 with 855 mm SVL + 196 mm TL = 1051 mm TTL, TL = 18.7% of TTL; AMS R.13302 with 1180 mm SVL with a truncated tail. PNGNM R25322 and SAMA R71443 exhibit dorsal scales in 17-19-15 rows while AMS R.13302 has a dorsal scale count of 19-19-15. Ventrals and subcaudals are, respectively, 231 and 83 (SCR = 0.26) in PNGNM R25322, 232 and 95 (SCR = 0.29) in SAMA R71443, and AMS R.13302 possesses 239 ventrals but the tail is truncated at 59 subcaudals. All three specimens have nine supralabials on either side, with SL4–SL5 contacting the eye, and nine infralabials on either side, with either IL1–IL4 or IL1–IL5 contacting the anterior genials.
There is some variation in the temporal scale arrangements in the four specimens. In the holotype the anterior and posterior upper temporals are distinct and separate, elongate scales, with the posterior scale slightly the larger ( Figs. 3 View FIGURE 3 A–B). The same state exists in the three paratypes but with some variation including fusion of these two scales into a single elongate upper temporal scale on the right side in PNGNM R25322 ( Fig. 3D View FIGURE 3 ), and on the left side in SAMA R71443 ( Fig. 3E View FIGURE 3 ). In AMS R.13302 the anterior upper temporal on the right side is almost twice the length of the posterior upper temporal ( Fig. 3H View FIGURE 3 ), the reverse of the holotype condition, while the upper posterior temporal on the left side is divided into two scales ( Fig. 3G View FIGURE 3 ). The lower temporal row of the holotype contains four scales on the left side, and five scales on the right side ( Figs. 3 View FIGURE 3 A-B). All three paratypes also exhibit either four or five scales in the lower temporal row, except PNGNM R25322 which possesses only two lower anterior temporals on the right side, the posterior-most of which comprises two scales fused to form a dumbbell shaped scale ( Fig. 3D View FIGURE 3 ). The temporal arrangement in S. aplini therefore comprises an elongate upper anterior temporal that may or may not be fused with the upper posterior temporal. When the upper anterior and posterior temporals are separate the anterior temporal is in contact with three lower temporals, when it is fused with the upper posterior temporal it is contacted by three or four lower temporals.
Colouration in life. The holotype and two paratypes were dorsally snow-white on the anterior and midbody regions, with infusions of black or very dark brown spots increasing in frequency from the midbody to posterior body. The dark pigment appears first on the posterior margins of the dorsal scales, gradually extending into the centres of each scale until by the tail the dorsum is primarily dark above, albeit still with large areas of white laterally. The venter is also immaculate white, with no dark pigmentation even posteriorly. The head is mid-brown dorsally, fading to light brown on the side of the head and on the neck. Although the colour in life of AMS R.13302 is unknown, its similarity in preservative to the remainder of the type series suggests a similar colouration in life.
Colouration in preservative. The holotype (SAMA R71442) is creamy-white anterior-dorsally, contrasting strongly with the dark brown dorsum of the head and neck. A few scattered dark brown spots are present on this pale background by midbody, from where they rapidly increase in frequency until the posterior body is dorsally reticulate, dark brown and white, and the dorsum of the tail is almost entirely brown, although white pigment persists on the lower dorsal scale rows. The venter is near-immaculate off-white to yellow throughout. The patterning of the paratypes is almost identical, although the head of AMS R.13302 is much paler brown possibly reflecting a much longer period in preservative.
Comparison with other species. Stegonotus aplini is a striking snake that can be immediately distinguished from all congeners with the exception of S. iridis by its colouration: at least the anterior third of the dorsal surfaces is snowy-white. All other congeners are unicolour grey, brown, or even black ( S. borneensis Inger, 1967 ), or have white, yellow, pink or orange-red upward-pointing triangles anteriorly with a distinctly pale head laterally ( S. batjanensis ), or a reticulated pattern across the dorsum ( S. reticulatus ). Our new taxon most closely resembles S. iridis from the Raja Ampat Archipelago ( Fig. 4 View FIGURE 4 ), but the two species can be distinguished by the following characters (characters of S. aplini in parentheses): 17-19-15, 71%, or 17-17-15, 29%, dorsal scale rows (17-19-15 rows, 75%, or 19-19-15 rows, 25%); ventrals 198–211 (229–239); subcaudals 78–88 (83–95); SCR 0.28–0.31 (0.26–0.29); supralabials usually eight (86%), occasionally nine (14%) (usually nine, 75%, occasionally eight, 25%); infrabials ten, with IL1–IL5 contacting the anterior genials (usually nine, 75%, or eight, 25%, with IL1–IL4 or IL1–IL5 contacting anterior genials). The temporal scale arrangement of S. aplini ( Figs. 3 View FIGURE 3 A–H) also differs from that of S. iridis ( Figs. 3 View FIGURE 3 I–J). Both species exhibit elongate upper anterior and posterior temporals and whilst both species may occasionally exhibit fragmentation of these scales to form three upper temporals, fusion of these scales into a single elongate upper temporal, extending the entire length of the parietals, has only been observed in two of the S. aplini paratypes (PNGNM R25322; SAMA R71443). The lower anterior temporal in S. iridis is elongate, resulting in only two lower temporals contacting the upper anterior temporal, while three, occasionally four, squarish lower temporals contact the upper anterior temporal in S. aplini . Stegonotus iridis also appears to be a smaller species, with males not known to exceed 845 mm SVL (three of the four known males of S. aplini exceed 1000 mm SVL). A list of the major features of scalation that distinguish these two species is presented in Table 1, and a dichotomous key to the fourteen species of Stegonotus currently recognised on New Guinea and two additional species from adjacent areas that may occur in southern New Guinea, is provided below.
Habitat and Natural History. Like other members of the genus Stegonotus , S. aplini is a nocturnal species that forages on the forest floor at night. This species was common in both primary lowland forest remote from human habitation ( Fig. 5 View FIGURE 5 ), and in garden regrowth near villages; at least ten individuals were observed during surveys of the Purari basin, where the species’ conspicuous pale colouration was readily visible in torchlight during night surveys. During capture each of the specimens struck repeatedly when handled, and attempted again to strike at researchers whilst being extracted from secure holding bags for examination. Local landowners greatly feared this species which they mistook for the highly venomous elapid Micropechis ikaheca 1 ( Lesson, 1830), the only other pale, nocturnal snake in the region. All known locations where this species has been located are in the lowlands of the Purari River basin ( Fig. 6 View FIGURE 6 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |