Grimaldina freyi, Neretina, Anna N. & Kotov, Alexey A., 2017
|
publication ID |
https://doi.org/10.11646/zootaxa.4291.2.4 |
|
publication LSID |
lsid:zoobank.org:pub:8A334FA5-F438-4539-86CB-8AA1924F3C3A |
|
DOI |
https://doi.org/10.5281/zenodo.6000295 |
|
persistent identifier |
https://treatment.plazi.org/id/03D7821F-FFB6-4C55-FF3A-A739EB4F468F |
|
treatment provided by |
Plazi |
|
scientific name |
Grimaldina freyi |
| status |
sp. nov. |
Grimaldina freyi sp. nov.
( Figs. 8–13 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11 View FIGURE 12 View FIGURE 13 )
Grimaldina brazzai Richard in Sars 1901 View in CoL : p. 28–31, pl. 5: figs. 1–14; Hollwedel et al. 2003: p. 78, figs. 10–40.? Grimaldina brazzai Richard in Kořínek 1984 View in CoL : pl. XXVI, fig. 1; Silva-Briano 1998: p. 151, figs. 9, 10; Garfias-Espejo & Elías- Gutiérrez, 2003: p. 108, figs. 1–8; Elías-Gutiérrez et al. 2006: p. 12–13, figs. 21–31; Fuentes-Reinés et al. 2012: fig. 12 a– d.
Etymology. This taxon is named in memory of Prof. Dr David G. Frey ( 10/10/1915 – 01/04/1992), one of the most famous cladoceran researchers, who proposed the modern methods of taxonomy, diversity and distribution in these microcrustaceans. The sample which was used for our description was collected by D.G. Frey.
Type locality. Lake Hicpochee ( N 26.79°, W 81.13°), Florida, U.S.A. GoogleMaps , North America. From this locality, over 50 parthenogenetic females were collected in 04/08/1960 by D.G. Frey.
Type material. Holotype. An adult parthenogenetic female preserved in 4% formaldehyde, deposited to the collection of Smithsonian National Museum of Natural History , Smithsonian Institution, U.S.A., USNM 1421862 About USNM . The holotype label reads: “ Grimaldina freyi sp. nov., 1 parth . ♀ from Lake Hicpochee , U.S.A., HOLOTYPE ” .
Paratypes. 10 undissected parthenogenetic females from Lake Hicpochee, U.S.A., coll. in 04/08/1960 by D.G. Frey, preserved in 4% formaldehyde and deposited to the collection of Smithsonian National Museum of Natural History, Smithsonian Institution, U.S.A., USNM 1421863 About USNM . 2 undissected parthenogenetic females from Lake Hicpochee, U.S.A., coll. in 04/08/1960 by D.G. Frey, preserved in 4% formaldehyde and deposited to the collection of Zoological Museum of M.V. Lomonosov Moscow State University, MGU Ml 153.
Other material studied (excluded from the type series). U.S.A.: 10 formaldehyde-fixed parthenogenetic females from a roadside pond, near Orange Lake, Florida, coll. in 17/04/1984 by D.G. Frey, DGF 6811; 4 formaldehyde-fixed parthenogenetic females from Georges Pond, Florida, coll. in 18/04/1984 by D.G. Frey, DGF 6814.
Mexico: 1 formaldehyde-fixed parthenogenetic female from Rancho San Juan, Tabasco, coll. in 13/10/1998 by N.N. Smirnov and M. Elías-Gutiérrez, AAK 2002-069; 1 formaldehyde-fixed parthenogenetic female from a water body in Quintana Roo, coll. in 04/07/2002 by M. Elías-Gutiérrez, J.G. Granados-Ramírez and A.A. Kotov, AAK 2002-040; 2 formaldehyde-fixed parthenogenetic females from a water body in Quintana Roo, coll. in 04/07/2002 by M. Elías-Gutiérrez, J.G. Granados-Ramírez and A.A. Kotov, AAK 2002-041; 1 formaldehyde-fixed parthenogenetic female from La Esperanza, cerca de Escarcega, Campeche, coll. in 13/10/1998 by N.N. Smirnov and M. Elías-Gutiérrez, AAK 2002-007.
Argentina: 1 parthenogenetic female from Santa Fe ( S 31.6°, W 60.6°), coll. in 02/05/1981 by N.N. Smirnov, NNS 1997-239. GoogleMaps
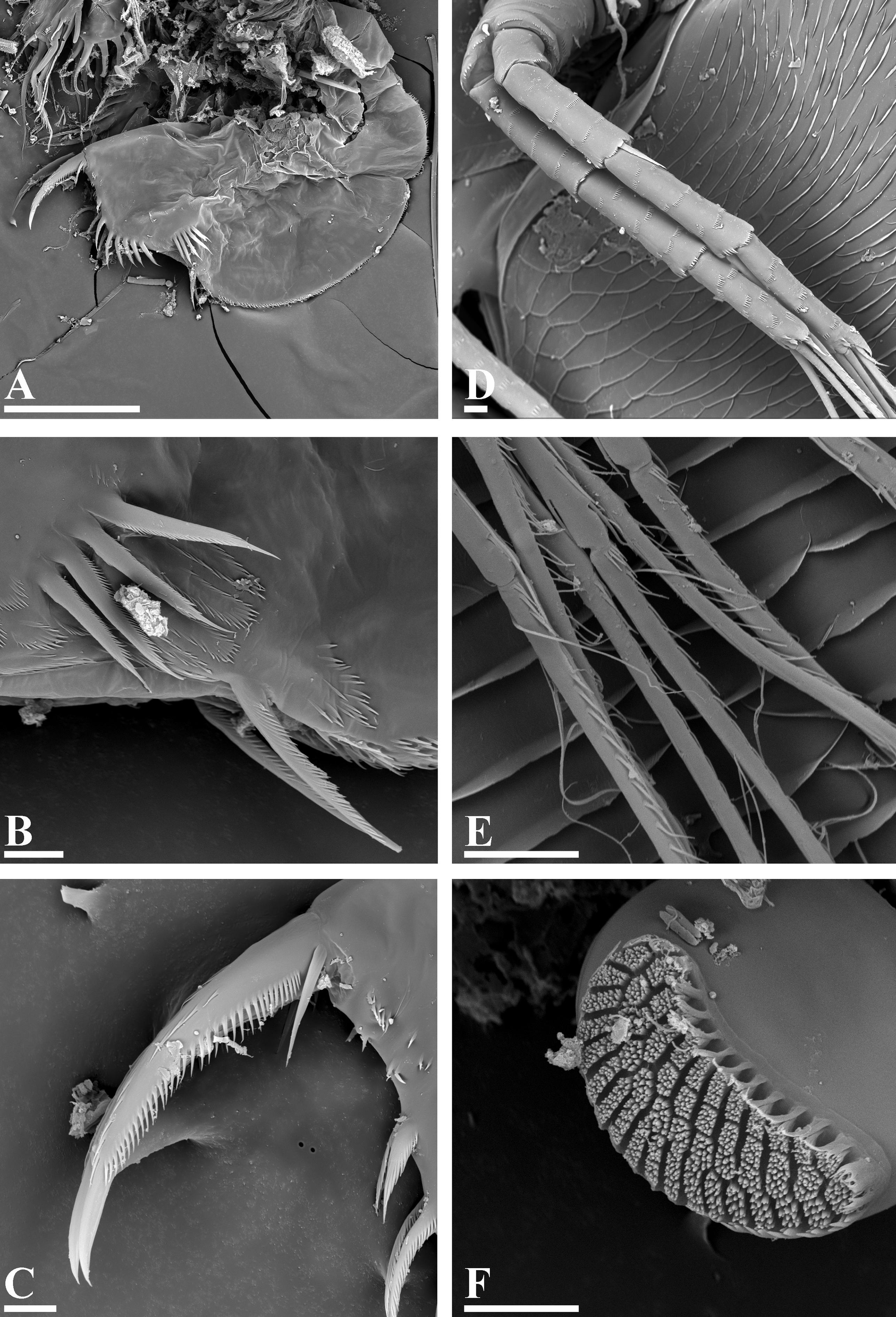
Description. Parthenogenetic female. General. In lateral view, body subrectangular, maximum height at middle of body (body height/length ratio 0.7 for adults) ( Figs 8 View FIGURE 8 A, 9A). In dorsal view body compressed laterally, without a dorsal keel. In anterior view, body elongated. Dorsal margin convex in anterior portion and straight in its posterior portion, depression between head and valves absent. Posterodorsal and posteroventral angles expressed, rounded. Anteroventral angle broadly rounded. Valves with prominent sculpture consisting of elongated scales ( Fig. 9 View FIGURE 9 A–F).
Head relatively large, not keeled, triangular in lateral view, with convex dorsal margin and strongly concave ventral margin ( Figs 8 View FIGURE 8 B, 9B–C); other details similar with previous species. A single frontal head pore ( Fig. 9 View FIGURE 9 D: arrow) located in anterior part of head between bases of antennae I. Dorsal head pores absent. Labrum massive, subquadrangular, with setulated apex and distal labral plate ( Fig. 8 View FIGURE 8 C).
Valve. Ventral margin regularly convex, covered by setulated setae as in G. brazzai , also slightly longer at posteroventral valve portion ( Fig. 8 View FIGURE 8 D–H). Inner side of posteroventral margin with short setulated setae, almost subequal in length ( Figs. 8 View FIGURE 8 H, 9F). On the inner side of posteroventral margin a row of small stiff setulae, increasing in size from posterodorsal angle to posteroventral angle ( Figs. 8 View FIGURE 8 I, 9G).
Thorax long, abdomen short.
Postabdomen large, subquadrangular, strongly compressed laterally ( Figs. 8 View FIGURE 8 J, 10A). Postabdomen length/ height ratio about 2. Ventral margin straight. Proportions and armature ( Figs 8 View FIGURE 8 J–L, 10A–B) similar to those in G. brazzai . Postabdominal setae longer than postabdomen, distal segment bearing long setulae. Postabdominal claw relatively robust ( Figs 8 View FIGURE 8 L–N, 10C), subequal in length to anal margin of postabdomen. External dorsal side covered in its proximal portion with a row of fine teeth, decreasing in size distally. Internal side of claw with a row of teeth, they slightly longer in proximal portion, and decreasing in size distally. Several teeth on ventral side of claw. On the base of claw, relatively long basal spine (more than two times longer than diameter of claw base) and a small denticle located proximally.
Antenna I long, thin, “rod like” ( Figs 9 View FIGURE 9 C, 11A), identical to G. brazzai . Nine aesthetascs, among them, seven aesthetascs subequal in length and two slightly longer.
Antenna II long ( Figs 10 View FIGURE 10 D, 11B). Antennal formula: setae 0-0-1-3/1-1-3, spines 0-1-0-1/0-0-1. Two sensory setae unequal in size at coxal part; basal segment cylindrical, covered by concentric rows of small denticles, bearing a small spine (almost subequal in length to basal exopod segment) and bisegmented setulated seta (slightly longer than basal endopod segment); all branches segments elongated, with concentric rows of small denticles. Exopod branch longer than endopod branch. Apical setae relatively short (shorter than antennal branches plus basal segment), feathered by long setulae and short stiff setulae ( Fig. 10 View FIGURE 10 E). The largest seta of proximal endopod segment asymmetrically armed by short stiff setulae (on proximal and distal portions) and more robust denticles (they cover distal portions and only slightly go to central portion) ( Fig. 11 View FIGURE 11 C–D). Distance between bases of spinulae almost two times longer than diameter of seta, measuring beside them (i.e. spinules on the longest endopod seta of antenna II located relatively densely). Seta of middle endopod segment and lateral seta of third exopod segment are identical to G. brazzai . Spine on second exopod segment thin, about three times shorter than third exopod segment. Spines of both apical segments thin, their proportions are similar to previous species.
Mandibles asymmetrical, molar surface of left mandible with numerous minute protuberances ( Fig. 10 View FIGURE 10 F).
Thoracic limbs: five pairs.
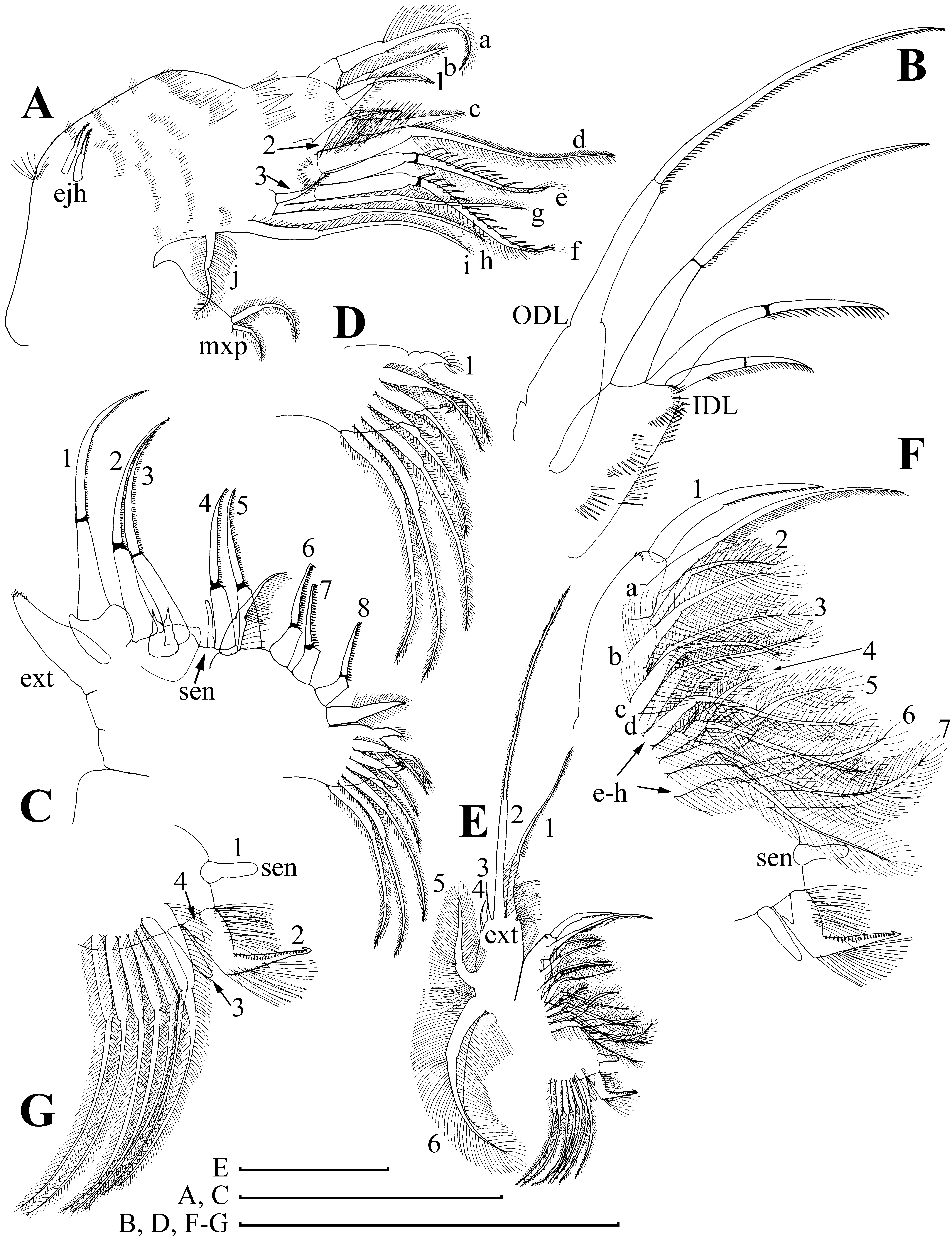
Limb I large ( Fig. 12 View FIGURE 12 A–B). Accessory seta not found. ODL conical, bearing a single long bisegmented seta, its distal segment setulated unilaterally ( Fig. 12 View FIGURE 12 B). IDL with three setae, different in length: the longest seta with fine setulae on its distal segment, the middle and short setae with fine denticles in the central and distal portions. Several groups of prominent setulae on IDL outer face. Limb corm rectangular in lateral view. Endite 4 ( Fig. 12 View FIGURE 12 A) with three posterior soft setae (seta “a” the longest with long fine setulae on its distal segment, setae “b” and “c” short, setulated) and a single stiff anterior seta. Endite 3 with three soft posterior setae unequal in size (among them seta “d” the longest, bearing fine setulae and setae “e” and “f” feathered unilaterally by rough setules) and a single stiff anterior seta (slightly longer than seta 1 of endite 4). Endite 2 with four posterior soft setae (setae g–i long, subequal in size, seta “j” significantly shorter, all setae covered by fine setulae) and a single stiff anterior seta (two times shorter than seta 2 of endite 4). All three stiff anterior setae with setulated distal segments. Endite 1, maxillar process, with two soft setulated setae, unequal in size. Two ejector hooks small, subequal in size. Concentric rows and bunches of fine setulae on ventral face of limb I.
Limb II triangular-rounded. Exopodite ( Fig. 12 View FIGURE 12 C: ext) ovoid, elongated, distally with minute setulae, without setae. Inner portion of limb II with eight scrapers (1–8), their proportions and armature identical to G. brazzai . Fork-like projection with bulbous base locates near scraper 3. A bisegmented sensillum and soft setulated seta are located near scraper 4. Also, soft setulated bisegmented seta near scraper 8. An incision between endite 2 and endite 1 (=gnathobase). Distalmost armature of gnathobase with four elements: three setae, unequal in size and armature (the distalmost seta short, bisegmented, with bulbose base, covered by fine setulae; the middle seta relatively long, thin, finely setulated; the proximal seta short, with stiff denticles) and a small bisegmented seta ( Fig. 12 View FIGURE 12 D). Filter plate bearing six setae, among them three distalmost setae short, and other three long.
Limb III with large ovoid epipodite, preepipodite not found. Exopodite ( Fig. 12 View FIGURE 12 E: ext) subrectangular, with two bilaterally setulated lateral setae unequal in size (5–6); and four distal setae (1–4): setae 3–4 short, setae 1–2 feathered by short setules in distal portions and bearing long setulae in proximal portions. Endite 5 bears a stiff anterior seta 1 and a long posterior seta “a” ( Fig. 12 View FIGURE 12 F). Endite 4 with a stiff seta 2 and a soft seta “b”. Endite 3 with a stiff seta 3 (a small sensillum near base of this seta) and two soft setae: “c” and “d”. Proximal endite with four anterior setae (4, 5, 6, 7; a minute sensillum near base of seta 4) and four posterior setae (e, f, g, h). Distal armature of gnathobase with a thick sensillum, two grouped sensillae and a single large densely setulated seta. Filter plate similar to previous species.
Limb IV with a large ovoid epipodite ( Fig. 13 View FIGURE 13 A: epp); preepipodite not studied. Exopodite rounded, with two long lateral setae (3–4) and two short distal setae unequal in size (1–2). Inner distal portion with four anterior setae ( Fig. 13 View FIGURE 13 B: 1–4) and a very large sensillum near seta 3. Posterior face with five soft setae (a–e). Distal armature of gnathobase and filter plate structure are identical to G. brazzai .
Limb V with rounded setulated preepipodite ( Fig. 13 View FIGURE 13 C: pep) and ovoid epipodite. Exopodite rounded, with a single long seta asymmetrically covered by rows of long and short setulae. Inner distal portion as a small setulated lobe; on its inner margin three setae significantly increasing in size distally: the longest seta clearly bisegmented, basal segment densely covered by fine setulae, distal segment with more widely spaced thick setulae; two other setae densely setulated. On inner distal portion, middle seta about two times longer than shortest seta (thus the proximalmost seta has middle size) ( Fig. 13 View FIGURE 13 D–E). Filter plate was not found, although at least three protuberances present.
Ephippial females and males. No ephippial females and males were present in our samples. See Sars (1901) for a description of the ephippium and male from South America.
Size. Maximum length of adult parthenogenetic females up to 0.71 mm; height 0.49 mm. Holotype is 0.70 mm in length and 0.49 mm in height.
Variability. No significant variability among New World specimens was found.
Differential diagnosis. Grimaldina freyi sp. nov. is generally similar to G. brazzai , but clearly different from it in fine morphological details: (1) more densely located spinules on the longest endopod seta of antenna II; (2) smaller ratio of seta 2 to seta 3 on inner portion of the limb V (for comparison see Table 1 and Fig. 14 View FIGURE 14 ).
Distribution. This species occurs in New World tropical regions, but it is obviously rare and not abundant in the water bodies where it is found. The range of G. freyi sp. nov. in Neotropics is reported from tropical South America ( Sars 1901; De Ferrato 1966; Brandorff et al. 1982; Silva-Briano 1998; Hollwedel et al. 2003; Fuentes- Reinés et al. 2012; Kotov & Fuentes-Reinés 2015; this study), through Central America ( Kořínek 1984; Elías- Gutiérrez et al. 1999; Garfias-Espejo & Elías-Gutiérrez 2003; Elías-Gutiérrez et al. 2006; Elías-Gutiérrez & Varela 2009; this study) and to subtropical regions of North America in southeastern U.S.A. (this study). There are several publications with more or less detailed descriptions and figures of G. freyi sp. nov. (as G. brazzai ) ( Sars 1901; Silva-Briano 1998; Garfias-Espejo & Elías-Gutiérrez 2003; Hollwedel et al. 2003; Elías-Gutiérrez et al. 2006; Fuentes-Reinés et al. 2012), and some features important for identification of this species are depicted ( Sars 1901; Garfias-Espejo & Elías-Gutiérrez 2003; Hollwedel et al. 2003).
We have revised Grimaldina and demonstrated the non-cosmopolitanism in its distribution, as it was previously made for some other tropical cladocerans (other examples include Rajapaksa & Fernando 1986; 1987a; b; Hudec 2000; Korovchinsky 2004; Kotov & Hollwedel 2004; Kotov et al. 2005; Neretina & Kotov 2015; Neretina & Sinev 2016). H. J. Dumont (personal communication) was the first author suggested that Grimaldina might include more than one species worldwide. The genus was found in South ( Chatterjee et al. 2013) and South East Asia ( Maiphae et al. 2008; Kotov et al. 2013; Korovchinsky 2013; Sinev & Korovchinsky 2013; Van Damme et al. 2013; Sinev & Yusoff 2015); as well as in Central (e.g. Elías-Gutiérrez et al. 2006) and South America (Hollwedel et al. 2003; Kotov & Fuentes-Reinés 2015). But an adequate understanding of the diversity in this genus was impossible due to incompleteness of Richard’s (1892) and subsequent (e.g. Sars 1901; Smirnov 1976; 1992) descriptions. Moreover, some authors ( Kořínek 1984; Silva-Briano 1998) mixed illustrations on specimens from the Old and New World in the same plates and ignored fine morphological differences, even with their doubts on the status of non-African populations. Grimaldina also remained unrevised due to a low numbers of samples. We demonstrate that G. brazzai and G. freyi sp. nov. parthenogenetic females are superficially similar, but clearly differ in some important fine features ( Table 1). Such traits of G. freyi sp. nov. as densely located endopod seta spinulae and longer proximalmost seta on limb V are presumably plesiomorphic.
The taxonomical value of fine morphological setal traits of the proximal endopod segment is demonstrated for another anomopods, ilyocryptids ( Kotov & Štifter 2006) and Macrothrix Baird, 1843 (see Silva-Briano 1998; Dumont et al. 2002; Kotov & Hollwedel 2004; Kotov et al. 2005). The ratio between lengths of setae on limb V is important in discrimination of some daphniids (e.g. Alonso 1996; Benzie 2005; Popova et al. 2016) and chydorids ( Kotov 2009), but has not been previously used in the macrothricids probably, due to insufficient study.
Fryer (1974: p. 237–238) noted for Grimaldina : “It has not been possible to study the feeding mechanism, but filtration of collected particles is certainly involved. The filter chamber, whose filtering surface are compressed by trunk limbs 3 and 4, is very well developed and the indications are that fine particles are collected. Thus the denticulation of the scrapers of trunk limb 2 is extremely fine and suggestive of sweeping rather than scraping. Its gnathobasic spines are long and slender, the anteriormost being modified for sweeping by a posterior row of fine spinules. Trunk limb 1 is relatively slender, which suggests great mobility, for which its musculature also appears suited, and the distal lobes bear slender claws. The use of these is enigmatic as they appear not to be specialized for dragging flocculent material as they are for example in Acantholeberis , but are not specialized for grasping. Further observations are required”. Therefore, Grimaldina feeding mechanism includes particles swept from the substrate and subsequent secondary filtering. The thoracic limbs are not used for scraping the substrate as in chydorids and they differ from the truly scraping limbs of Macrothrix (i.e., thoracic limbs I–II with less prominent setal armature, and thoracic limbs III–V more delicate ( Sars 1901; Fryer 1974)).
Grimaldina View in CoL has diversified and relatively long valve setae, which are convergently similar to those in truly benthic anomopods such as Ilyocryptus Sars, 1862 View in CoL ( Fryer 1974; Kotov & Dumont 2000; Kotov & Štifter 2006) or Leydigia Kurz, 1875 ( Kotov 2009) View in CoL . At the same time, the former (in contrast to the later two genera) "appear not to be a burrower" ( Fryer 1974, P. 236). A.Y. Sinev (personal communication) collected most abundant samples with Grimaldina brazzai View in CoL from lake areas overgrown with Pistia View in CoL sp. or Salvinia View in CoL sp., with apparent oxygen deficiency and hydrogen sulfide smell, and these animals were mostly collected from the plants roots, not immediately from the bottom. It is interesting that living Grimaldina View in CoL specimens studied by Sars (1901) were of a reddish brown color, which is also typical for benthic Ilyocryptus View in CoL and Leydigia View in CoL . The reddish body color in benthic cladocerans is explained by a string hemoglobin production, an adaptation to oxygen deficiency ( Smirnov 2014).
The rarity of Grimaldina View in CoL records could partly be explained by a specific mode of life. Typical cladoceran collections consist of plankton samples only. Thus, few authors had ample samples of Grimaldina View in CoL (e.g. Garfias- Espejo & Elías-Gutiérrez 2003; Elías-Gutiérrez et al. 2006).
Grimaldina View in CoL occurrence and abundance may be also associated with a particular season or rainfall events. It is common that standard faunistic samplings performed by European researchers in tropical regions are made during the dry seasons in order to avoid diseases such as malaria. Remarkably, Grimaldina View in CoL prefers small puddles, swampy grasslands and pools with vegetation, rather than rocky or sandy littoral of large lakes (e.g. Sars 1901; Thomas 1961; Rey & Saint-Jean 1968; Elías-Gutiérrez et al. 2006; Van Damme et al. 2013; this study). Generally, efforts are aimed at lake zooplankton, while tropical marshy areas and swamps remain poorly investigated and future studies are needed (e.g. Van Damme et al. 2013).
Cladocerans probably diversified in the mid-Paleozoic ( Fryer 1995; Dumont & Negrea 2002; Kotov & Korovchinsky 2006; Van Damme & Kotov 2016), passed through several extinction bottlenecks (Korovchinsky 2006), and now are successful and diverse across continental water bodies ( Dumont & Negrea 2002; Kotov 2013). Apparently, many cladocerans were differentiated before the Gondwana breakup. The ancestors of cladocerans presumably inhabited some shallow water bodies and had unspecialized benthic life modes (see details in Kotov 2013). With this in mind, the macrothricid-like organization is plesiomorphic as compared with a chydorid-like organization specialized to life in some particular littoral zone niches. It is possible that the Grimaldina View in CoL species originated from a common ancestor having a Gondwanian distribution range.
But the Old-New World differentiation could be a result of a long-distance dispersal events and subsequent speciation. Continental endemism concept is based on the fact that, although many cladocerans species are easily distributed by different vectors (including water birds) (Kotov 2013; Rogers 2014), for them it is rather difficult to form stable populations in new habitats already occupied by other taxa, better adapted to local conditions ( Rogers 2015). Therefore there is no mixing of different faunas (e.g. De Meester et al. 2002). Among cladocerans several well-documented examples of human-mediated invasions are known, but they concern mainly more evolutionary advanced forms such as daphniids ( Mergeay et al. 2005; Kotov & Taylor 2014) and chydorids ( Sharma & Kotov 2014). To date, there are no confirmed examples of invasive macrothricids. But intercontinental transitions apparently took place in some other Cladocera groups (e.g. Adamowicz et al. 2009). Since we have no molecular data for Grimaldina from different regions and paleontological records of macrothricids are unknown, we do not know which theory is best.
During the last two decades, distribution of many tropical groups of animals, plants and fungi were reconsidered based on new molecular and paleontological data, and different scenarios for Paleotropical- Neotropical differentiation were revealed. Vascular plants, especially angiosperms, with circumtropical distribution are the most actively studied group due to a large number of paleontological records and expanded genetic information (e.g. Antonelli et al. 2009; Li & Wen 2013; Nie et al. 2013). Post-Boreotropical dispersals for studied angiosperm plants explain their contemporary distribution patterns, and, at least for some species, cases of dispersal events from the Old World to New World and vice versa were convincingly demonstrated ( Antonelli et al. 2009; Li & Wen 2013; Nie et al. 2013). The same distribution patterns were found in some ectomycorrhizal fungi, associated with circumtropical vascular plants (e.g. Harrower et al. 2015). Boreotropical migration was considered as the most possible scenario for the explanation of current distribution of the cladoceran genus Leydigiopsis Sars, 1901 ( Van Damme & Sinev 2013) . Among other crustaceans, a Gondwanian origin was not confirmed for freshwater shrimps ( Page et al. 2005), prawns ( Murphy & Austin 2005) and crabs ( Daniels et al. 2006). Dispersal events, occurring long after the split up of Gondwana, explain contemporary distribution patterns of many taxa. However, studies on freshwater crayfish diversification supported the Gondwana origin hypothesis. Moreover, the differentiation of crayfish superfamilies was associated with the division of Pangaea ( Crandal et al. 2000). It should be taken into consideration that sampling (the number of investigated taxa) and the estimation of paleontological age (calibration scale) strongly influence the results of such analysis. If the fossil crayfish are known from Permian and Early Triassic deposits (see links in Crandal et al. 2000), the fossil findings for freshwater crabs are relatively recent, with the oldest known from the upper Miocene ( Daniels et al. 2006). For some other ancient groups, such as freshwater sponges, the worldwide geographic range suggests a Gondwana biographic pattern ( Manconi et al. 2015). Recently a complex situation was shown in tropical diving beetles: a potential Gondwana origin of some groups and later trans-Pacific dispersal took place towards South America in the Oligocene ( Toussaint et al. 2016). The explanation of contemporary distribution patterns for tropical organisms, including the cladocerans, remains an interesting and intriguing task for future investigations.
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Grimaldina freyi
| Neretina, Anna N. & Kotov, Alexey A. 2017 |
Leydigia Kurz, 1875 ( Kotov 2009 )
| Kurz, 1875 (Kotov 2009 |
Grimaldina brazzai Richard in Kořínek 1984
| Richard in Korinek 1984 |
Grimaldina brazzai
| Richard in Sars 1901 |
Ilyocryptus
| Sars 1862 |