Lysapsus, Cope, 1862
|
publication ID |
https://doi.org/10.1206/0003-0090(2005)294[0001:SROTFF]2.0.CO;2 |
|
persistent identifier |
https://treatment.plazi.org/id/03D887A5-FFD2-891E-FD67-FA0ACF96F9EF |
|
treatment provided by |
Felipe |
|
scientific name |
Lysapsus |
| status |
|
Lysapsus View in CoL , Pseudis , and Scarthyla
The sistergroup relationship between Scarthyla goinorum and ‘‘pseudids’’ ( Lysapsus 1 Pseudis ), and this group being nested within Hylinae , corroborates recent findings by Darst and Cannatella (2004) and Haas (2003). Burton (2004) reported a likely synapomorphy for the Scarthyla plus the ‘‘pseu did’’ clade that is corroborated in the present analysis; that is, the m. transversus metatarsus II oblique, with a narrow, proximal connection to metatarsus II, and a broad, distal connection to metatarsus III. Another character state described by Burton (2004), the undivided tendon of the m. flexor digitorum brevis superficialis, optimizes in this analysis as a synapomorphy of this clade plus Scinax .
Besides the molecular data, the monophyly of Lysapsus plus Pseudis is further supported by the tendo superficialis pro digiti III arising from the m. flexor digitorum brevis superficialis, with no contribution from the aponeurosis plantaris; the origin of m. flexor ossis metatarsi IV and the joint tendon of origin of mm. flexores ossum metatarsorum II and III crossing each other; the m. flexor ossis metatarsi IV very short, inserting on the proximal twothirds of metatarsal IV or less; absence of a tendon from the m. flexor digitorum brevis superficialis to the medial slip of the medial m. lumbricalis brevis digiti V; and m. transversus metatarsus III oblique, with a narrow, proximal connection onto metatarsal III, and a broad, distal connection to metatarsal IV. Another likely morphological synapomorphy is the elongated intercalary elements.
Vera Candioti (2004) noticed that Lysapsus limellum and most of the 30chromosome Hyla ( H. nana and H. microcephala ) studied by her and Haas (2003) share two of the synapomorphies that Haas (2003) reported for Pseudis paradoxa and P. minuta : insertion of the m. levator mandibulae lateralis in the nasal sac, and a distinct gap in the m. subarcualis rectus II–IV. Haas (2003) observed different character states for H. ebraccata (the m. levator mandibulae lateralis inserts in tissue close to posterodorsal process of suprarostral cartilage or adrostral tissue; continuous m. subarcualis rectus II–IV), suggesting the need for additional studies on its taxonomic distribution within the 30chromosome Hyla and in the other genera of the South American II clade.
Sphaenorhynchus , Xenohyla and the 30 Chromosome Hyla
Izecksohn (1959, 1996) suggested possible relationships of Xenohyla with Sphaenorhyn chus and Scinax (Izecksohn, 1996) . These ideas are partially corroborated by our 1:1:1 results, with the exception that they also suggest that Xenohyla is the sister group of the 30chromosome Hyla . The karyotype is still unknown in Xenohyla , and this poses an obstacle to our understanding of the limits of the 30chromosome Hyla . Interestingly, while both Sphaenorhynchus and Xenohyla do have a quadratojugal, in both cases it does not articulate with the maxilla (Duellman and Wiens, 1992; Izecksohn, 1996), which could be seen as an intermediate step before the extreme reductions of the quadratojugal seen in the 30chromosome Hyla (Duellman and Trueb, 1983) . Tadpoles of Xenohyla and several species of 30chromosome Hyla share the presence of the tail tip extended into a flagellum, as well as the presence of high caudal fins (e.g., see Bokermann, 1963; Kenny, 1969; Gomes and Peixoto, 1991a, 1991b; Izecksohn, 1996; Peixoto and Gomes, 1999).
Phylogenetic hypotheses of the 30chromosome Hyla species groups using morphological characters were presented by Duellman and Trueb (1983), Duellman et al. (1997), Kaplan (1991, 1994), and Kaplan and Ruíz (1997); none of these tested the monophyly of the contained species groups. A summary of their proposals and the supporting evidence are depicted in figure 6 View Fig .
Chek et al. (2001) presented a phylogenetic analysis using partial 16S and cytochrome b sequences of the Hyla leucophyllata group, including exemplars of other 30 chromosome Hyla species groups. Because they did not include non30chromosome hylids, they did not test the monophyly of this clade.
The distribution of certain characters in several species associated with the currently recognized species groups suggests problems in our phylogenetic understanding of these frogs. The monophyly of a group composed of the Hyla leucophyllata , H. marmorata , H. microcephala , and H. parviceps groups is currently supported by the absence of labial tooth rows in their larvae (Duellman and Trueb, 1983). However, within the H. parviceps group, H. microps (Santos et al., 1998) and H. giesleri (Bokermann, 1963; Santos et al., 1998) have at least one labial tooth row. Similarly, Gomes and Peixoto (1991a) and Peixoto and Gomes (1999) noticed in the H. marmorata group the presence of one labial tooth row in the larvae of H. nahdereri , H. senicula , and H. soaresi . Based on these facts and similarities in tail depth, tail color, general body shape, and predatory habits, they suggested that the H. marmorata group could instead be more closely related to H. minuta than to the groups suggested by Duellman and Trueb (1983). Gomes and Peixoto (1991b) pointed out the presence of a labial tooth row in the larva of H. elegans . Wild (1992) further noted the absence of marginal papillae (an apparent synapomorphy of the H. microcephala group) in the larva of H. allenorum (a species of the H. parviceps group).
The reproductive modes of the different species are also informative. According to Duellman and Crump (1974), Hyla parviceps deposits its eggs directly in the water, as does H. microps (Bokermann, 1963) , whereas H. bokermanni and H. brevifrons oviposit on leaves overhanging ponds; upon hatching, the tadpoles drop into the water where they complete development. Hyla ruschii oviposits on leaves overhanging streams (Weygoldt and Peixoto, 1987). The oviposition on leaves occurs in most species of the H. leucophyllata group, whereas both reproductive modes occur in the H. microcephala group.
Our results recover the 30chromosome Hyla species as monophyletic; however, our topology differs from previous hypotheses. In our topology, the root is placed between the H. marmorata group and the other exemplars, instead of between the H. labialis group and the other exemplars, as was assumed in previous analyses (Duellman and Trueb, 1983; Kaplan, 1991, 1994, 1999; Chek et al., 2001).
Topological differences from previous hypotheses are not due merely to a rerooting of the previously accepted tree; the relationships obtained by our analysis are quite different from previous proposals. Our analysis does not recover as monophyletic the exemplars of the three species groups once thought to be monophyletic on the basis of lacking labial tooth rows, that is, the Hyla leucophyllata , H. microcephala , and H. parviceps groups. Instead, the H. microcephala group (including the taxa imbedded within it) is the sister taxon of a clade composed of H. anceps and the exemplars of the H. leucophyllata group. The exemplars of the H. parviceps group are the sister taxon of a clade composed of the exemplars of the H. columbiana and H. labialis groups. This shows that the scenario of labial tooth row evolution is more complex than previously thought, because it implies several transformations in both directions between presence and absence of labial teeth within the clade.
Observations by Wassersug (1980), Spirandeli Cruz (1991), and Kaplan and Ruiz Carranza (1997) on the internal oral features of larvae of representatives of the Hyla leucophyllata ( H. ebraccata and H. sarayacuensis ), H. microcephala ( H. microcephala , H. nana , H. phlebodes , and H. sanborni ), and H. garagoensis ( H. padreluna and H. virolinensis ) groups revealed a reduction of internal oral structures (including reduction of most internal papillation, reduction of branchial baskets, reduction or absence of secretory ridges and secretory pits) that is most extreme in the representatives of the H. microcephala group. Hyla minuta does not show the reductions seen in these species groups (Spirandeli Cruz, 1991). This species also shares with representatives of the H. leucophyllata group described by Wassersug (1980) a reduction in the density of the filter mesh of the branchial baskets in comparison with other hylid tadpoles. It is clear that the study of internal oral features will provide several additional characters relevant for the study of the 30chromosome species of Hyla .
The exemplars of the Hyla parviceps group obtain as monophyletic. However, we included only 3 of the 15 species currently included in this problematic group. We are not confident that the monophyly of the H. parviceps group will be maintained as more taxa are added. Regarding the exemplars of the H. leucophyllata group, their relationships are equivalent to those obtained by Chek et al. (2001).
The sistergroup relationship of Hyla anceps and the H. leucophyllata group corrob orates early suggestions by Lutz (1948, 1973) that these could be related on the basis of sharing a large axilar membrane and flash coloration.
While we could not test the monophyly of the Hyla minima and H. minuta groups, our exemplars of these groups are sister taxa, and they are only distantly related with the exemplars of the H. parviceps group. This position does not support Duellman’s (2001) tentative suggestion that the species of the H. minima group should be included in the H. parviceps group.
The paraphyly of the Hyla microcephala group with respect to the H. rubicundula group is an expected result, as historically its species were associated with H. nana and H. sanborni (Lutz, 1973) . Nevertheless, the association of the H. microcephala and H. rubicundula groups were reinforced by Pugliese et al. (2001), who described the larva of H. rubicundula and noted similarities (like the lack of marginal papillae) with the larvae of members of the H. microcephala group. In particular, these authors noticed similarities with H. nana and H. sanborni , with the latter being the sister taxon of H. rubicundula in our analysis.
Carvalho e Silva et al. (2003) segregated the Hyla decipiens group from the H. microcephala group on the basis that the larvae of these species lack the possible morphological synapomorphies currently diagnostic of the H. microcephala group (body of tadpole depressed, labial papillae absent in tadpoles) and the putative clade composed of the H. leucophyllata , H. microcephala , and H. parviceps groups (absence of labial tooth rows). However, as mentioned above, our results imply a complex scenario for labial tooth row transformations and place the H. decipiens group within the H. microcephala group. The available taxon sampling did not allow testing the monophyly of the H. decipiens group. The fact that its known species share the oviposition on leaves above the water, and the reversals in larval morphology that led Carvalho e Silva et al. (2003) to consider them unrelated to the H. microcephala group, probably indicates that, even if nested inside this group, the species assigned to the H. decipiens group could be a monophyletic unit.
The relationships of the Hyla garagoensis group, from which no exemplar was available for this study, were discussed by Kaplan and RuizCarranza (1997). Based on the absence of labial tooth rows, they placed the H. garagoensis group in a polytomy together with the H. marmorata group and the clade composed of the H. microcephala , H. parviceps , and H. leucophyllata groups. Duellman et al. (1997) presented a cladogram for most of the 30chromosome Hyla groups, where the H. garagoensis and H. marmorata groups appear together as a clade supported by the presence of one ventral row of small marginal papillae in larvae. This character state needs further assessment, as indicated by Gomes and Peixoto (1991a) and Peixoto and Gomes (1999), because tadpoles of the H. marmorata group have either one ( H. nahdereri ) or two rows of marginal papillae ( H. senicula ; H. soaresi ); the tadpoles of H. padreluna , a species of the H. garagoensis group, also has a double row (Kaplan and RuizCarranza, 1997). Considering this and earlier comments, we do not see evidence that associates the H. garagoensis group with the H. marmorata group more than with any other group within the 30chromosome Hyla clade.
MIDDLE AMERICAN/HOLARCTIC CLADE
This clade is composed of most of the Middle American/Holarctic genera and species groups of treefrogs ( fig. 5). For the purposes of discussion, we divide it into four large clades. The first of these includes Acris and Pseudacris . The second includes Plectrohyla , the Hyla bistincta group, the H. sumichrasti group, and various elements of the H. miotympanum group. The third clade includes Duellmanohyla , Ptychohyla , H. miliaria , H. bromeliacia (the sole exemplar of the H. bromeliacia group), and one element of the H. miotympanum group. The fourth clade includes Smilisca , Triprion , Anotheca , and the exemplars of the H. arborea , H. cinerea , H. eximia , H. godmani , H. mixomaculata , H. pictipes , H. pseudopuma , H. taeniopus , and H. versicolor groups.
Within the Middle American/Holarctic clade, the genera Ptychohyla and Smilisca , as well as the Hyla arborea , H. cinerea , H. ex imia, H. miotympanum , H. tuberculosa , and H. versicolor groups, are not monophyletic. The H. miotympanum group is polyphyletic; its exemplars split among three different clades: H. miotympanum is the sister taxon of one of the exemplars of the H. tuberculosa group, H. miliaria ; H. arborescandens and H. cyclada are related to an undescribed species close to H. thorecte s, and together are related to exemplars of the H. bistincta group; H. melanomma and H. perkinsi are at the base of the H. sumichrasti group. Smilisca is not monophyletic, having Pternohyla fodiens nested within it. Ptychohyla is paraphyletic with respect to H. dendrophasma ( H. tuberculosa group). The H. arborea group is polyphyletic, with H. japonica nest ed within the H. eximia group. The H. cinerea group is not monophyletic, with H. femoralis being more closely related to members of the H. eximia and H. versicolor groups than to H. cinerea , H. gratiosa , and H. squirella . The H. versicolor group is not monophyletic because H. andersonii is more closely related to the H. eximia group.
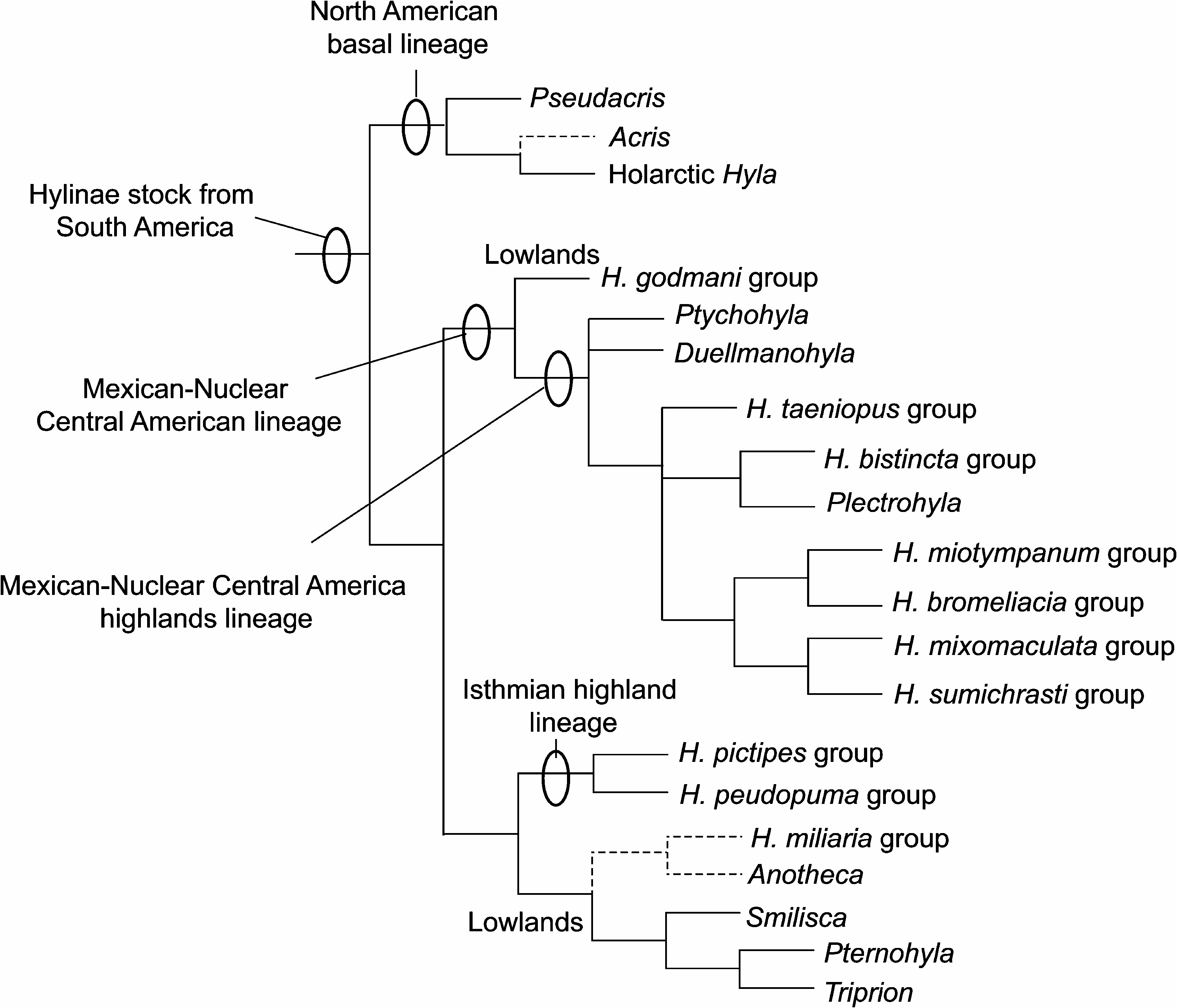
The monophyly of all genera and species groups of Hyla contained in this clade was maintained by Duellman (1970, 2001) based mostly on biogeographic grounds, because morphological evidence of monophyly was lacking. Duellman (2001) further presented a diagram depicting ‘‘suggested possible evolutionary relationships’’ among Middle and North American Hylinae , using as terminals the species groups of Hyla and the different genera (redrawn here as fig. 7 View Fig ). Duellman (2001) envisioned a North American basal lineage being the sister taxon of what he called the Middle American basal lineage. This Middle American basal lineage is further divided into a lower Central American lineage (itself divided into an isthmian highland lineage and a lowland lineage) and the MexicanNuclear Central American lineage (in turn divided into a MexicanNuclear Central American highland lineage and a lowlands lineage).
While the basal position of Acris and Pseudacris in this clade is consistent with Duellman’s (2001) intuitive suggestion of a North American basal lineage, it differs in that the Holarctic species groups of Hyla are only distantly related to them.
(2001). Broken lines are tentative placements.
We found no evidence supporting Duellman’s (2001) exclusively MexicanNuclear Central American lineage, nor of his MexicanNuclear Central American highland lineage. The latter has nested within it the MexicanNuclear Central American lowland clade (the H. godmani group), a clade reminiscent of Duellman’s (2001) isthmian highlandlowlands lineage, and also all species groups of Holarctic Hyla . Biogeographic implications of the discordant nature of our results with Duellman’s suggested relationships between Middle and North American Hylinae will be dealt with from a biogeographic perspective later in this paper.
The nonmonophyly of North American Hylinae does not agree with previous analyses (Hedges, 1986; Cocroft, 1994; da Silva, 1997; Moriarty and Cannatella, 2004) because those analyses assumed implicitly that Acris , the Holarctic Hyla , and Pseudacris are monophyletic. In spite of this, the internal relationships of Pseudacris recovered in this analysis are consistent to those obtained by Moriarty and Cannatella (2004).
Previous analyses either did not find evidence that Acris was particularly close to any group of North American hylids (Cocroft, 1994), or else suggested relationships with different species groups of North American Hyla (Hedges, 1986; da Silva, 1997). Two morphological synapomorphies of this Acris 1 Pseudacris clade could be the spherical or ovoid testes (as opposed to elongate testes) and the presence of dark pigmentation in the peritoneum surrounding the testes (Ralin, 1970, as cited by Hedges, 1986).
The nonmonophyly of the Hyla miotympanum group is not surprising because, as discussed in the section on taxon sampling, it did not appear that any of its putative synapomorphies could withstand a test with a broader taxon sampling. Duellman (2001) merged the formerly recognized H. pinorum group (Duellman, 1970) with the H. miotympanum group. With the notable exception of H. miotympanum , our results are close to recovering both groups as originally envisioned by Duellman (1970), because H. cyclada and H. arborescandens ( H. miotympanum group) are recovered as monophyletic, and the former H. pinorum group is recovered as a paraphyletic assemblage that includes the H. sumichrasti group nested within it. In his analysis (Duellman, 2001: 912), the two species included by Duellman (1970) in the H. pinorum group ( H. melanomma and H. pinorum ), plus the two species that were later associated with this group ( H. perkinsi and H. juanitae ), form a monophyletic group supported by a single synapomorphy, the presence of an extensive (equal to or more than onehalf length of upper arm) axillary membrane.
The dubious monophyly of the 17 species assigned to the Hyla bistincta group was not seriously tested in our analysis, because only 2 species were available. These two exemplars form the sister group of two taxa previously associated with the H. miotympanum group, and together they form the sister taxon of Plectrohyla . According to Duellman and Campbell (1992), character states supporting a monophyletic H. bistincta group plus Plectrohyla are: medial ramus of pterygoid long, in contact with otic capsule; dorsal skin thick (but see Mendelson and Toal [1995] and Duellman [2001] for discussions of this character); complete marginal papillae of the oral disc; and presence of at least one row of submarginal papillae (called by these authors accessory labial papillae) on the posterior labium (but see Wilson et al. [1994a] for discussion of this character state). From these, the complete marginal papillae of the oral disc occur in all known larvae of the clade containing Plectrohyla , and the H. bistincta , H. sumichrasti , and fragments of the H. miotympanum group, as well as in larvae of several other nearby clades ( Ptychohyla , Duellmanohyla , the H. mixomaculata , and H. taeniopus groups; see Duellman 1970, 2001). Furthermore, the row of submarginal papillae in the larval oral disc presents a fair amount of variation in the extent and distribution of the papillae, within which could probably be subsumed the morphology seen in all known larvae of the clade mentioned above (see illustrations of all these oral discs in Duellman, 1970, 2001). Besides discussions provided by Mendelson and Toal (1995) and Duellman (2001) regarding the definition of the character state ‘‘thick skin’’, it does not occur in the following species currently assigned to the H. bistincta group: H. calvicollina , H. charadricola , H. chryses , H. labedactyla , and H. sabrina (Duellman, 2001) . Considering that 15 of 17 species currently included in the H. bistincta group and 15 of 18 included in Plectrohyla could not be included in the analysis, we do not consider our results a strong test of their intrarelationships, particularly when several species of the H. bistincta group that present suspicious character state combinations, like the ones mentioned above, were not available. Our results relating H. arborescandens with species of the H. bistincta group corroborate earlier suggestions by Caldwell (1974) that relate this species to species currently placed in the H. bistincta group ( H. mykter , H. robertsorum , and H. siopela .). Mendelson and Toal (1996) also suggested affinities of H. arborescandens and H. hazalae with the H. bistincta group on the basis of unpublished osteological data.
Duellman (2001) noted the lack of evidence for the monophyly of the Hyla tuberculosa group. Although poor, our taxon sampling does not recover it as monophyletic, because H. dendrophasma is nested within Ptychohyla , and H. miliaria is the sister taxon of H. miotympanum . Duellman (2001) also referred to the possibility advanced by da Silva (1997) of a relationship of the H. tuberculosa group with the Gladiator Frogs; at this point the evidence presented herein does not support this idea, but in case a dens er sampling of the group still corroborates its polyphyly, we would not be surprised if some of its elements (particularly H. tuberculosa 26) are shown to be related with the TGF clade.
Hyla miotympanum has repeatedly been considered a generalized Middle American hyline (Duellman, 1963, 1970; Campbell and Smith, 1992; Duellman, 2001), largely because the larva of H. miotympanum exhibits a labial tooth row formula of 2/3 and a relatively small oral disc with an anterior gap in the marginal papillae. We are not aware of any morphological synapomorphy supporting its relationship with H. miliaria , although our molecular data firmly place it there. According with our results, there is a morphological synapomorphy supporting the monophyly of the clade composed of these two species plus Duellmanohyla , the H. bromeliacia group, and Ptychohyla (including H. dendrophasma ): the tendo superficialis hallucis that tapers from an expanded corner of the aponeurosis plantaris, with fibers of the m. transversus plantae distalis originating on distal tarsal 2–3 that insert on the lateral side of the tendon.
Considering its overall external appearance, we are surprised by the position of the poorly known Hyla dendrophasma . This species was originally considered to be a member of the H. tuberculosa group (Campbell et al., 2000) based on its large snout–vent length and extensive hand webbing, although with the caveat that it lacks dermal fringes, the only character state shared by all other species placed in the H. tuberculosa group. DNA was isolated and sequenced twice from tissues of the female holotype, the only known specimen (Campbell et al., 2000). Inasmuch as most previous notions of relationships among species of Ptychohyla derive from adult male morphology and tadpoles, the discovery of at least one male specimen of H. dendrophasma could hopefully allow
26 The other South American species of the Hyla tuberculosa group, H. phantasmagoria , is known only from the holotype. It was considered a junior synonym of H. miliaria by Duellman (1970), who later resurrected it (Duellman, 2001). Besides a few comments by this author, no morphological comparisons with other species of the group are available.
us to better understand its relationships within Ptychohyla .
Campbell and Smith (1992) and Duellman (2001) suggested five morphological synapomorphies for Ptychohyla . One of these is apparently unique to Ptychohyla (pars palatina of the premaxilla with welldeveloped lingual flange), while the other four show a more extensive taxonomic distribution. (1) The cluster of ventrolateral mucous glands in breeding males is present in Duellmanohyla chamulae , D. ignicolor , and D. schmidtorum (Campbell and Smith, 1992; see also Thomas et al., 1993). (2) The presence in the ventrolateral edge of forearm of tubercles coalesced into a ridge (as opposed to the absence of tubercles) was reported for D. lythrodes , D. salvavida , D. schmidtorum , and D. soralia (Duellman, 1970; 2001); H. bromeliacia has an indistinct row of tubercles that do not coalesce into a ridge (absent in H. dendroscarta ) (Duellman, 1970). (3) The double row of marginal papillae is present as well in larvae of the H. bromeliacia group (Duellman, 1970). Finally, (4) larvae of the H. bromeliacia group have a labial tooth row formula of 2/4 or 2/5, and all known larvae of Duellmanohyla have a labial tooth row formula of 3/3; the minimum known for a species of Ptychohyla is 3/5 ( P. legleri and P. salvadorensis ) (Duellman, 1970, 2001; Campbell and Smith, 1992).
The monophyly of the group composed of Ptychohyla euthysanota , P. hypomykter , P. leonhardschultzei , and P. zophodes is congruent with the results of Duellman (2001), who supported the monophyly of these taxa based on the presence of a thick, rounded tarsal fold. These species further share the presence of hypertrophied ventrolateral glands in breeding males with two species we could not include in our analysis: P. macrotympanum and P. panchoi . Furthermore, all these species also share with P. spinipollex the presence of the nuptial excrescences composed of enlarged individual spines. The states of these characters are unknown in Ptychohyla sp. and Hyla dendrophasma because the only available specimens are females. The nonmonophyly of P. hypomykter plus P. spinipollex is most surprising, considering that both were considered to be a single species (Wilson and McCranie, 1989; see also McCranie and Wilson, 1993).
Although we included several species of Ptychohyla in our analysis, we do not think that we have apprehended a good representation of the morphological diversity of the group, and the absence of species like P. erythromma , P. legleri , and P. sanctaecrucis certainly weakens the test of monophyly of Ptychohyla . This is more so considering the fact that several of the putative morphological synapomorphies of Ptychohyla are actually shared with some species of its sister taxon, as discussed earlier, and that the monophyly of our exemplars of Ptychohyla is weakly supported. The low Bremer support (3) for Ptychohyla also suggests that the evidence for its monophyly deserves further attention.
The Hyla bromeliacia group was tentatively associated with the polyphyletic H. miotympanum group by Duellman (2001: 779). Other than this, we are not aware of it being associated with any other group. Besides the molecular evidence, we are aware of at least one likely morphological synapomorphy supporting the monophyly of Duellmanohyla plus the Hyla bromeliacia group: the presence of pointed serrations of the larval jaw sheaths (Campbell and Smith, 1992; Duellman, 1970; 2001). These are apparently longer in some species of Duellmanohyla than in the H. bromeliacia group, but both seem to be notably more pointed than in Ptychohyla (see descriptions and illustrations in Duellman, 1970).
We share with Duellman (2001) and Mendelson and Campbell (1999) doubts regarding the monophyly of the Hyla taeniopus group. Nevertheless, our two exemplars are recovered as monophyletic in the analysis. Duellman (2001) examined the possibility of a relationship between this group and the H. bistincta group, based on the fact that both have large streamadapted tadpoles with small, ventral oral discs with complete marginal papillae and bear a labial tooth row formula of 2/3 (but noting that the toothrow formula is slightly higher for H. nephila and H. trux ). Our results suggest instead that the H. taeniopus group is the sister taxon of a clade composed of H. mixe (the only available exemplar of the H. mixomaculata group) plus the clade composed of the Holarctic Hyla groups, the H. godmani , H. pictipes , and H. pseudopuma groups, and Anotheca , Smilisca (including Pternohyla ), and Triprion . Furthermore, in the context of our results, the ventral oral disc with complete marginal papillae seems to be a synapomorphy of the whole Middle American clade, with subsequent transformations in the clade just mentioned and in other points of the tree.
We were unable to test the monophyly of the Hyla mixomaculata group, because H. mixe was the only taxon available. Regardless, and until a rigorous test is possible, the monophyly of this group could be reasonably assumed based on the presence of the enlarged oral disc with 7/10 or 11 labial tooth rows. At this point it should be stressed that the sequenced sample comes from a tadpole that was assigned to the H. mixomaculata group based that on that characteristic, and that it was tentatively assigned to H. mixe for being the only species of the group known from the region where the larva was collect ed; thus, considering the uncertainty in its determination, its position in the tree should be viewed cautiously.
Duellman (2001) included the species of the former Hyla picta group in the H. godmani group. Unfortunately, the two exemplars available to us for this analysis are only the two members of the former H. picta group and none of the restricted H. godmani group; therefore, this is not a satisfactory test of the monophyly of the H. godmani group (sensu lato).
The Lower Central American Lineage
The monophyly of the included exemplars of the Hyla pictipes and H. pseudopuma groups, and its relationship with a clade composed of Anotheca , Smilisca , Triprion , and Pternohyla , is quite consistent (in the sense that it contains almost the same groups) with Duellman’s intuitive proposal of a lower Central American clade that contains an Isthmian Highland lineage and a Lowland lineage ( fig. 7 View Fig ), with the only exception being that he tentatively considered the H. miliaria group related to Anotheca .
The monophyly of a group composed of Hyla pseudopuma and H. rivularis , the only two exemplars available from the H. pseudopuma and H. pictipes groups, could be suggestive of a lineage of highland isthmian Hylinae as suggested by Duellman (2001). Although the monophyly of each of these two groups has not been tested here, the position of the undescribed Mexican species Hyla sp. 5 (aff. H. thorectes ) deserves some comments.
Duellman (1970) recognized the Hyla hazelae group in which he included the nominal species and H. thorectes . Reasons for recognizing this group were ‘‘the combination of large hands with vestigial webbing, half webbed feet... and presence of a tympanum are external features which separate these species from other small streambreeding Mexican Hyla . Furthermore both species have small, relatively narrow tongues and large tubercles below the anal opening. The nature of the nasals and sphenethmoid are unique among northern Middle American hylids’’ (Duellman, 1970: 384). It is unclear if any of these character states could have been considered as evidence of monophyly of the group. It is also unclear on what basis Duellman (2001) dismantled the group and placed H. hazelae in the H. miotympanum group, while H. thorectes was transferred to the H. pictipes group. Wilson et al. (1994b) suggested that H. thorectes could be related to H. insolita and H. calypsa (under the name H. lancasteri ; see Lips, 1996) because they share oviposition on leaves overhanging streams and have dark ventral pigmentation; these character states were not included in Duellman’s (2001) analysis of the group. Although we do not have data to take a position regarding these actions, the fact that Hyla sp. 5 (aff. H. thorectes ) is unrelated to H. rivularis is here taken as evidence that H. thorectes should not be included in the H. pictipes group. Furthermore, all character states advanced by Duellman (2001) as shared by H. thorectes and the species of the H. pictipes group are also shared by H. thorectes and the taxa to which Hyla sp. 5 (aff. H. thorectes ) appears to be closely related in our analysis. Because we were unable to include H. calypsa , H. insolita , or H. lancasteri , we do not have elements to test the hypothesis of Wilson et al. (1994b) regarding the close re lationship of H. calypsa , H. insolita , and H. thorectes .
In order to better understand the relationships of the Hyla pictipes group, it would be important to add to this analysis exemplars of the former H. zeteki and H. lancasteri groups, because together with the original H. pictipes and H. rivularis groups (as defined by Duellman, 1970) they represent the three main morphological extremes of the group. The fact that so few exemplars of these two groups were available is one of the weaker points of our analysis.
The paraphyly of Smilisca is partly consistent with Duellman’s (2001) phylogenetic analysis of this genus in that Pternohyla is nested within it. However, we did not recover Triprion nested within Smilisca , as did Duellman (2001).
A possible relationship between Triprion and Anotheca was first advanced by Lutz (1968) because she considered them to be the extreme of one specialization consisting ‘‘in excessive ossification of the head, accompanied at some stages by extra dentition’’ (Lutz, 1968: 10). Within this same line, she included all casqueheaded frogs, including together South American and Middle American forms. Duellman and Trueb (1976) suggested a possible link of Anotheca with Nyctimantis (discussed below). Duellman (2001: 332) proposed a tentative relation of Anotheca with the Hyla miliaria group based on the oophagous tadpoles that develop in bromeliads or treeholes (known for the only species of the H. tuberculosa group with a known tadpole, H. salvaje ; see Wilson et al., 1985). While our results support a sistergroup relationship of Anotheca and Triprion as suggested by Lutz (1968), it occurs within the Holarctic/Middle American clade and not within a group composed of all casquehead ed frogs as she suggested. In the context of this analysis, both Triprion and Anotheca share the posterior expansion of the frontoparietals that cover almost all the otoccipital dorsally (see figures in Duellman, 1970).
Our analysis indicates that the insertion of m. extensor digitorum comunis longus on metatarsal II is a synapomorphy of a group composed of Anotheca , Triprion , and the paraphyletic Smilisca . Furthermore, Smilisca (including Pternohyla ) and Triprion share the type I septomaxillary (see Trueb, 1970a) and bifurcated cavum principale of the olfactory capsule (Trueb, 1970a); the distribution of these character states should be studied in Anotheca and nearby groups to determine the level of inclusiveness of these possible synapomorphies.
Real Hyla
Our results concerning the relationships between the North American and Eurasiatic species groups of Hyla differ from previous analyses, in part likely because of the previous assumption of monophyly of North American/Holarctic Hylinae . In the first place, the molecular evidence supports a clade containing all North American and Eurasiatic species groups of Hyla , a result that differs from previous analyses where relationships either were unresolved (Cocroft, 1994) or were paraphyletic with respect to Acris and/or Pseudacris (Hedges, 1986; da Silva, 1997). The polyphyly of the Hyla arborea group and the paraphyly of the H. eximia group corroborate previous ideas by Anderson (1991) and Borkin (1999) regarding their nonmonophyly and the closer relationship of H. japonica with the H. eximia and H. versicolor groups. A likely synapomorphy of the H. eximia and H. versicolor groups, including H. japonica and H. andersonii , is the nucleolar organizer region (NOR) present in chromosome 6 instead of chromosome 10 (Anderson, 1991).
SOUTH AMERICAN/WEST INDIAN CASQUE HEADED FROGS
This clade ( fig. 5) is composed of Phyllodytes , Phrynohyas , Nyctimantis , and all South American/West Indian casqueheaded frogs: Argenteohyla , Aparasphenodon , Corythomantis , Osteopilus , Osteocephalus , Trachycephalus , and Tepuihyla . It is divided basally in a group composed of the two exemplars of Phyllodytes , and another group composed of all casqueheaded frog genera, including Phrynohyas and Nyctimantis .
Within this clade, other than those genera that are not monotypic ( Argenteohyla , Nyctimantis , Corythomantis ) or represented in this analysis by a single species ( Aparasphenodon , Tepuihyla ), Phyllodytes and Os teopilus are monophyletic, and Osteocephalus , Phrynohyas , and Trachycephalus are not monophyletic. Osteocephalus is not monophyletic because O. langsdorffii (the only species of the genus distributed in the Atlantic forest) is not related to the remaining exemplars of Osteocephalus , which form a monophyletic group that is the sister taxon of Tepuihyla . Phrynohyas is not monophyletic, having Trachycephalus nigromaculatus nested within it, and Trachycephalus is not monophyletic, with T. jordani forming the sister taxon of ‘‘ Phrynohyas ’’ 1 T. nigromaculatus .
With respect to Phyllodytes , we were unable to find any published hypothesis regarding its relationships, and considering the scant information available on its morphology, we had no previous clue as to other groups of Hylidae with which it might be related. The only morphological character state of which we are aware that Phyllodytes shares with several members of the South American/West Indian Casqueheaded Frogs clade is the presence of at least four posterior labial tooth rows in the tadpole oral disc (see below, ‘‘Taxonomic Conclusions: A New Taxonomy of Hylinae and Phyllomedusinae’’, for further details).
The polyphyly of Osteocephalus was not unexpected considering the lack of any evidence of its monophyly. This polyphyly results because of the position of O. langsdorffii . This is the only species of the genus present in the Atlantic forest and a species that had been particularly poorly discussed in the context of the systematics of Osteocephalus (Duellman, 1974) . While we do not test the monophyly of the bromeliadbreeding/single vocal sac species (here represented by a O. oophagus ), our results show that our exemplars with lateral vocal sacs are paraphyletic with respect to O. oophagus .
The monophyly of Tepuihyla was not test ed in this analysis. Its sistergroup relationship with Osteocephalus (excluding O. langsdorffii ) is supported by our data, instead of with Scinax as first suggested (Ayarzagüena et al., ‘‘1992’’ [1993b]; see earlier discussion on the relationships of Scinax ). This situation requires changes in the original interpretation of two character states that provided evidence of the relationships of Te puihyla with other hylids. The presence of spicules in the dorsum of males is more parsimoniously interpreted as a putative synapomorphy of Tepuihyla plus Osteocephalu s, instead of a homoplasy, as advanced by Ayarzagüena et al. (‘‘1992’’ [1993b]). Similarly, the reduction of webbing between toes I and II is more parsimoniously interpreted as a putative synapomorphy of Tepuihyla (homoplastic with Scinax ) instead of a synapomorphy of Scinax 1 Tepuihyla .
The monophyly of the four exemplars of Osteopilus corroborates in a much broader taxonomic context the results of Maxson (1992), Hedges (1996), and Hass et al. (2001), based on albumin immunological distances and still unpublished sequence data regarding its monophyly, and the recent taxonomic changes summarized by Powell and Henderson (2003b). Unfortunately, we could not include in our analysis O. marianae , O. pulchrilineatus , and O. wilderi , and we are not aware of any possible morphological synapomorphy supporting their monophyly. In the absence of other evidence, it could be suggested that the oviposition and development in bromeliads, which occurs in these three species and O. brunneus , is a possible synapomorphy uniting these with O. crucialis , which apparently also has this reproductive mode (Hedges, 1987).
The presence of paired lateral vocal sacs and biogeographic considerations led Trueb (1970b) and Trueb and Duellman (1971) to suggest the collective monophyly of Argenteohyla , Osteocephalus , Phrynohyas , and Trachycephalus (at that time the species of Osteocephalus having a single subgular vocal sac were still unknown). Furthermore, these authors considered Trachycephalus and Phrynohyas to be a monophyletic group on the basis of sharing vocal sacs that are more lateral and protrude posteriorly to the angles of the jaws when inflated. Trueb and Tyler (1974) suggested that the West Indian Osteopilus and the former Calyptahyla crucialis (now Osteopilus crucialis ) were also related to this clade, although they exhibit a single, subgular vocal sac. Our results corroborate the monophyly of Phrynohyas plus Trachycephalus (see below), but they also suggest a more complex situation where the casqueheaded frogs with double vocal sacs are par aphyletic, with all of the genera of casqueheaded frogs that have a single subgular sac being nested within them.
Duellman and Trueb (1976) suggested that Nyctimantis was related to Anotheca , because both share the medial ramus of the pterygoid being juxtaposed squarely against the anterolateral corner of the ventral ledge of the otic capsule. Also, frogs of both genera are known ( Anotheca ; Taylor, 1954; Jungfer, 1996) or suspected ( Nyctimantis ; Duellman and Trueb, 1976) to deposit their eggs in waterfilled tree cavities. Our results suggest a radically different picture, with Nyctimantis nested within the South American/West Indian Casqueheaded Frogs, while Anotheca is nested within the Middle American/Holarctic clade, being the sister taxon of Triprion .
The topology has some discrepancies with previous suggestions as to the relationships of Argenteohyla , Aparasphenodon , and Corythomantis (for the latter two genera, see also comments for Scinax above). Argenteohyla siemersi was segregated by Trueb (1970b) from Trachycephalus , where it had been placed by Klappenbach (1961), because it lacks the diagnostic character states of Trachycephalus established by Trueb (1970a). Further, she suggested that Argenteohyla is a close ally of Osteocephalus based on the presence of paired lateral vocal sacs. Although Trueb (1970a) suggested that Aparasphenodon and Corythomantis are sister taxa, our evidence suggests that both Argenteohyla and Nyctimantis are closer to Aparasphenodon than to Corythomantis .
Most species in the South American/West Indies Casqueheaded Frog clade frequently live in or seek refuge in bromeliads or treeholes. This has been reported for Aparasphenodon (Paolillo and Cerda, 1981; Teixeira et al., 2002), Argenteohyla (Barrio and Lutz, 1966; Cespedez, 2000), Corythomantis (Jared et al., 1999) , Nyctimantis (Duellman and Trueb, 1976) , Osteocephalus langsdorffii (Haddad, personal obs.), Phrynohyas (Goeldi, 1907; Prado et al., 2003), Tepuihyla (Ayarzagüena et al., ‘‘1992’’ [1993b]), and Trachycephalus (Lutz, 1954; Bokermann, 1966c). Furthermore, all species of Phyllodytes (Peixoto et al., 2003) , some species of Osteocephalus (Jungfer and Schiesari, 1995; Jungfer and Weygoldt, 1999; Jungfer and Lehr, 2001), some species of Osteopilus (Hedges, 1987; Lannoo et al., 1987), and at least two species of Phrynohyas (Goeldi, 1907; Lescure and Marty, 2000) even lay their eggs in phytotelmata or treeholes where their exotrophic larvae develop. While bromeliads and treeholes are used as refuges or for reproduction in other groups of hylids (e.g., Anotheca spinosa , the Hyla bromeliacia group, the two bromeliad breeding frogs of the H. pictipes group, H. astartea , the H. tuberculosa group, Scinax alter , the Scinax perpusillus group of the S. catharinae clade), the South American/West Indian Casqueheaded Frog clade seems to be the largest clade of hylids that consistently makes use of bromeliads or treeholes.
The phylogenetic structure of the South American/West Indies Casqueheaded Frog clade implies a minimum of one instance of reversal from presence of heavily exostosed and coossified skulls to normal looking, albeit heavily built skulls (the case of Phrynohyas ), and at least a possible second and third instance involving reversals from exostosed skulls (the cases of Tepuihyla and Osteopilus vastus ).
From a morphological perspective, the paraphyly of Phrynohyas with respect to Trachycephalus nigromaculatus and the concomitant nonmonophyly of Trachycephalus are most interesting and surprising. Herpetologists have been noticing for years that T. nigromaculatus and Phrynohyas mesophaea produce hybrids throughout their overlapping ranges of distribution (Haddad, personal obs.; Pombal, personal commun.; Ramos and Gasparini, 2004). The possibility of hybridization leads us to think that perhaps the introgression of P. mesophaea mitochondria could actually be the reason for the recovered paraphyly of Trachycephalus . However, phylogenetic analyses using either tyrosinase or rhodopsin alone (the only two nuclear genes that were successfully sequenced in T. nigromaculatus ) still recover a paraphyletic Trachycephalus (results not shown).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.