Zorion Pascoe
|
publication ID |
https://doi.org/ 10.5281/zenodo.170224 |
|
DOI |
https://doi.org/10.5281/zenodo.6264952 |
|
persistent identifier |
https://treatment.plazi.org/id/03D8B618-182C-FF9E-431C-F945FB1A202F |
|
treatment provided by |
Plazi |
|
scientific name |
Zorion Pascoe |
| status |
|
Genus Zorion Pascoe View in CoL
Zorion Pascoe, 1867: 310 View in CoL .— Pascoe, 1869: 423; Broun, 1880: 584; Aurivillius, 1912: 156; Blair, 1937: 265; Kuschel, 1982: 3; Klimaszewski & Watt, 1997: 65, 161, 180; Grant, 1999: 97. [Type species: Callidium minutum Fabricius 1775 View in CoL ].
Distribution. Endemic to New Zealand.
External structures
Body shape. Slim, elongate with posterior region of prothorax forming a distinct waist separating thorax from elytra.
Head. Head wider than prothorax. Width about as long as height; constricted at base; central and frontal sutures clearly visible. Distance between lower lobes of eyes as long as distance between outer margins of antennal sockets.
Eyes. Strongly emarginate, with fine facets. Lower lobe almost round, much larger than the narrow elongate upper lobe which is almost separated from the lower lobe and connected only by a single or double row of omatidia.
Antennae. Filiform, about as long as body in both sexes. Antennal tubercles clearly raised; antennal sockets circular and laterally outward facing. Scape longest, widening apically; pedicel as long as wide; segments 1>5>3>4>6>7>8=11>9>10>2; scape, pedicel and segments 3–8 sparsely covered with erect hairs; segments 8–11 increasingly more pubescent.
Prothorax. Almost 2 as long as wide and divided into three areas. Anterior area almost cylindrical but restricted, and longer, wider and higher than cylindrical restricted posterior area; middle area with two lateral nodes longer, wider and higher than anterior area, and pronotum with 2–8 dorsal hairs. Prosternum slightly rugose; anterior coxal cavities closed and situated in middle and posterior region of prothorax. Prosternal process reduced to a thin ridge between the contiguous coxae; front coxae globose. Anterior and posterior areas of pronotum with dark edge.
Mesothorax. Scutellum triangular with a longitudinal black central suture at base. Mesocoxal cavities open laterally, separated by a narrow mesosternal process; coxae protruding, conical and almost contiguous.
Metasternum. Width about 0.8 of length of meso and metathorax combined. Metacoxae separated, slightly transversely, and slightly protruding.
Abdomen. Five visible segments, ovate, entirely covered by elytra, sparsely covered in fine hairs on ventral side.
Legs. ( Figs. 1–3 View FIGURES 1 – 3 ). Length of mesthoracic legs 1.25 length of prothoracic legs; length of metathoracic legs 1.5 length of prothoracic legs; length of prothoracic legs 0.58 of total body length. Femura either dark or light coloured or light coloured with dark ring. Femora club shaped ( Figs. 1–3 View FIGURES 1 – 3 ). Tibiae elongate, as long as corresponding femora, with two spurs at apex of equal length at protibiae, inner meso and metatibiae spurs longer than outer ones. Femora covered with sparse short hairs; tibiae covered dorsally with short hairs, becoming more pubescent ventrally.
Elytra ( Figs. 4–5 View FIGURES 4 – 5 ). Elongate with a pointed to rounded apex. Shoulders raised to a higher point dorsally and laterally. Elytral length about 0.66 of body length and about 2 body width across humeri. Central axis through elytral spot at forward, right or backward angle in relation to axis along elytral margin ( Figs. 4–5 View FIGURES 4 – 5 ). Female with hairs ( Fig. 5 View FIGURES 4 – 5 ) on anterior epipleural fold not reaching beyond spots on elytra, male without such hairs ( Fig. 4 View FIGURES 4 – 5 ). Colour and spot patterns on elytra variable.
Terminalia
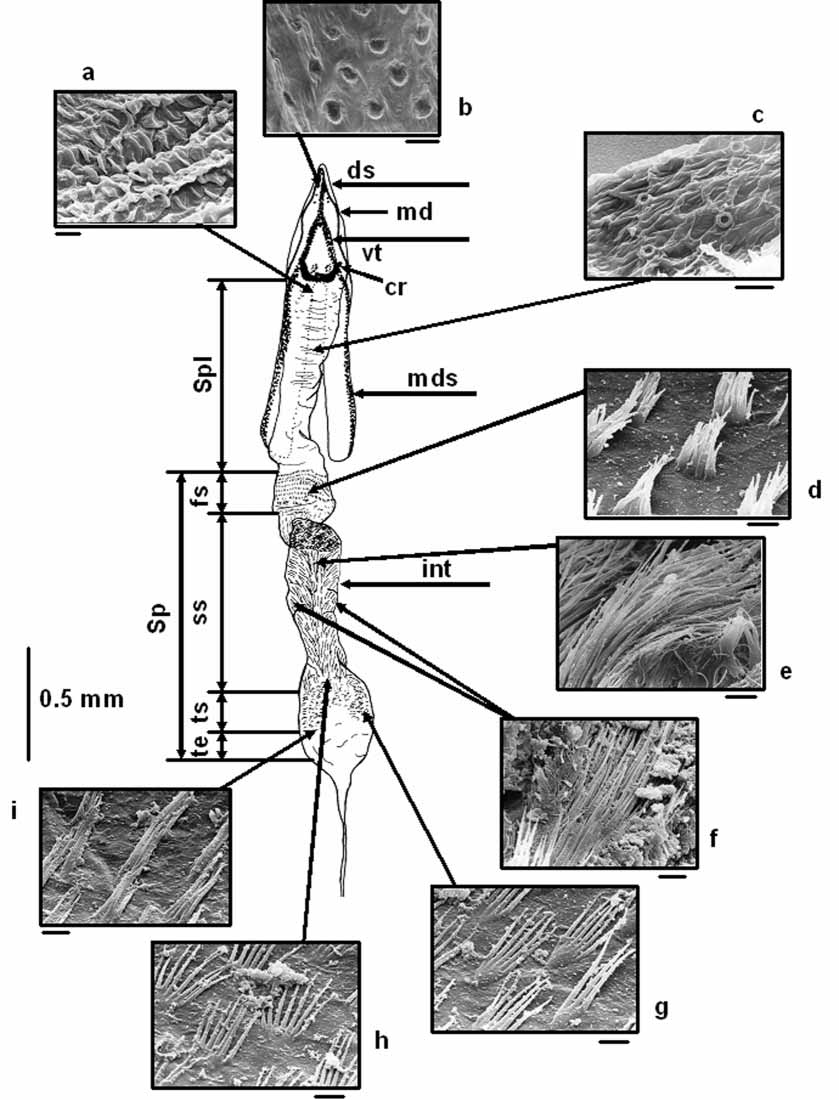
Male terminalia: We could not find significant differences in male terminalia between Zorion species. SEM images of some interior spines of the aedeagus are depicted in Fig. 6 View FIGURE 6 , showing the different spines present in the internal sac of the aedeagus.
Aedeagus ( Fig. 6 View FIGURE 6 ). Apex of median lobe pointed, widening into a bulbous base, narrower behind bulb and widening again towards median struts. Internal sac divided into two regions: basal spineless and terminal spinose. Spineless region transparent and appearing with a scale like surface around area of chitinous rods half way down median struts. Spinose region longer than spineless region. Spinose region divided into 4 sections, first section short starting at end of median struts, with fine thin short hairlike spines arranged in grouped rows ( Fig. 6 View FIGURE 6 d); second section 4 as long as third, with dense long thin single spines ( Fig. 6 View FIGURE 6 e) in median area, and sparse paired spines in lateral area ( Fig. 6 View FIGURE 6 f), becoming smaller and turning into paired spines and even smaller multiple spines; third section with scale like digitate spines ( Fig. 6 View FIGURE 6 g–i) ending in a fourth short transparent section ( Fig. 6 View FIGURE 6 ).
Eighth sternite ( Fig. 7 View FIGURES 7 – 9 ). Apex emarginate, forming two lobes with microspines and long hairs.
Eighth tergite ( Fig. 8 View FIGURES 7 – 9 ). Apex rounded or truncate and almost rectangular shaped, with fine hairs at apical edge and single pointed microspines in mid area turning gradually into scale like spines towards lateral margins.
Tegmen ( Fig. 9 View FIGURES 7 – 9 ). Consisting of parameres that are two lobed, a spinate, transparent roof and a ringed part; spines multipointed.
Female terminalia: Including fifth tergite, eighth sternite, ovipositor, bursa copulatrix and spermatheca.
Fifth tergite (Fig. 10). Obovate, truncate at base, covered with microspines forming two iridescent eyes, apex with short and long hairs.
FIGURES 10–12. Female terminalia of Zorion . 10, 5th tergite scales on forming two iridescent eyespots(es) in females. 11, 8th sternite: sp, spinate section and spines; sc, scaled section and scales. 12, ovipositor; vb, ventral baculi; db, dorsal baculi. Scale bars Figs. 10 and 11, 0.25mm; Fig. 12, 0.5 mm.
Eighth sternite (Fig. 11). Bilobate, scattered with multipointed spines strongly sclerotised at point; spines gradually changing into less sclerotised and scaleshaped towards apex.
Ovipositor (Fig. 12). Divided into two sections, first section with broad scales becoming slimmer towards the second section; second section with very fine hairlike spines or scales. Dorsal baculi shorter than ventral baculi; dorsal baculi arising from base of coxite, ventral baculi arising from between 0.16 to 0.12 times the dorsal baculi length up from base of coxite. Coxite with stronger sclerotisation on inner sides. Base of coxite with small sclerotised rods. Styli sclerotised, flattened at apex with three simple large and long spines; styli slightly pointing outwards.
General Biology
Feeding: The beetles feed on pollen from a variety of flowers, preferably on small flowers that are arranged into an inflorescence such as Hebe (Scrophulariaceae) , Pomaderris (Rhamnaceae) or wild carrot. Their feeding behaviour may contribute to the pollination of plants. Even though beetles are mainly found on shrubby plants in edge communities or open landscape, females might venture farther into the forest to find host plants for oviposition. Specimens were collected in mature forest at Lake Papaitonga (Levin) (Melissa Hutchison, pers. comm.). Kuschel (1990) lists Zorion habitat to be in canopy as well as in bush but gives no records on canopy flowers. It needs to be investigated whether the beetles visit flowers in the canopy and hence may be important pollinators of New Zealand trees.
Appearance: The body outline of the genus Zorion resembles that of Hymenoptera and it is suggested that the genus is an ant mimic (J. Dugdale, G. Kuschel, pers. comm.). Yet, when we collected Zorion we observed that the golden spots on the elytra resembled the yellow pollen sacs of native bees.
Mating: Crossmating between species of collected Zorion guttigerum and Z. australe beetles did not result in any oviposition. All Z. guttigerum beetles that we collected in late September and early October were found as single individuals on flowers and did not mate for several days after collected, whereas beetles collected later varied in numbers between 2 and 5 individuals on one florescence and readily mated when placed into a Petri dish. Wang (2002) gives an account on the sexual selection of Z. guttigerum in relation to the colour and body size of individuals.
Laboratory breeding: We dissected Z. guttigerum pupae and larvae from Alnus cordata (Betulaceae) twigs collected in midSeptember. Larvae were supplied with a diet for Cerambycid woodboring larvae (Rogers et al. 2001) and kept at 20ºC with a photoperiod of 16L:8D. The larvae soon pupated and all pupae eclosed in midOctober.
We kept some collected Z. guttigerum adults in plastic cylinder containers (65mm diameter by 83mm high) supplied with Physocarpus opulifolius (Rosaceae) flowers as a food source. Mating took place within the container, and eggs were deposited in notches cut into the bark of Alnus cordata twigs about 1cm diameter and 5cm length. Larvae hatched after 1 to 2 weeks and soon started tunnelling under the bark. Rapid growth could be observed but the larvae died after several weeks. Newly hatched larvae reared on a Cerambycid diet did not survive. Dumbleton (1957) reared adults from larvae found in hawthorn, Sequoia gigantae (Taxodiaceae) and flax ( Phormium tenax ( Agavaceae )) and Kuschel (1990) found Z. batesi ovipositing in Eucalyptus sp. ( Myrtaceae ). Zorion species use a wide variety of exotic and native plants as hosts for oviposition. Milligan (1975) named Zorion amongst several other Cerambycinae species, which feed between the bark and the sap wood or occupy small branches or lianes soon after death of the host. They share this habitat with other cerambycid species, and other woodboring families. Dumbleton (1957) provided a key to the larvae of Cerambycinae including Zorion and gave a description of Z. minutum larvae from the Nelson area, which we believe were most likely Z. australe larvae. He could not distinguish the larvae of the two species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Zorion Pascoe
| Schnitzler, Franz-Rudolf & Wang, Qiao 2005 |
Zorion
| Grant 1999: 97 |
| Klimaszewski 1997: 65 |
| Kuschel 1982: 3 |
| Blair 1937: 265 |
| Aurivillius 1912: 156 |
| Broun 1880: 584 |
| Pascoe 1869: 423 |
| Pascoe 1867: 310 |