Dendromonocotyle bradsmithi, Chisholm, Leslie A., Glennon, Vanessa & Whittington, Ian D., 2005
|
publication ID |
https://doi.org/10.5281/zenodo.171185 |
|
DOI |
https://doi.org/10.5281/zenodo.6264441 |
|
persistent identifier |
https://treatment.plazi.org/id/03DA0305-FFFE-FF9E-FEED-A53EFCAB8A89 |
|
treatment provided by |
Plazi |
|
scientific name |
Dendromonocotyle bradsmithi |
| status |
sp. nov. |
Dendromonocotyle bradsmithi View in CoL n. sp.
Typehost: Myliobatis australis Macleay, 1881 (Myliobatidae) .
Typelocality: Port Adelaide River ( 34°48’00’’S, 138°31’00’’E), off Torrens Island. Outer Harbour, Adelaide, South Australia, Australia.
Other localities: Comet Bay ( 32°25'68"S, 115°43'83"E) off Mandurah, Western Australia and The Aquarium of Western Australia (AQWA), Perth, Western Australia, Australia.
Site: Dorsal skin surface.
Infection details: Seven rays infected with numerous parasite specimens. Prevalence 100%.
Etymology: The species is named after Mr Brad Smith, a keen angler, who caught the rays in South Australia.
Material deposited: Holotype SAMA AHC 28776, 24 paratypes SAMA AHC 28777 28800; 18 paratypes USNPC 95896 and 95897.
Adult description (Figs 1–3)
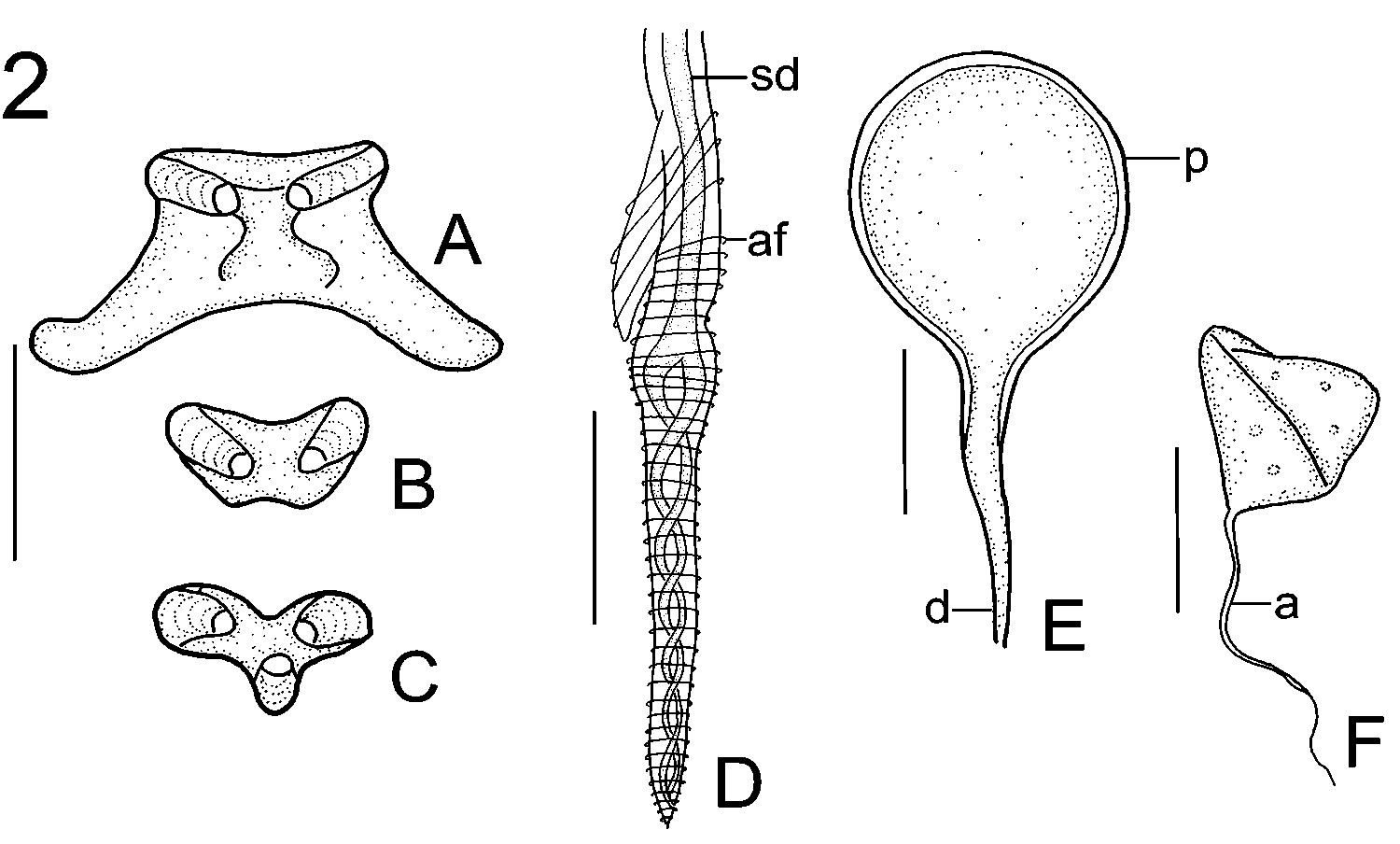
Dendromonocotyle ( sensu Chisholm & Whittington 1995). Description and measurements based on 2 live and 25 fixed and mounted sexually mature specimens. Live adult worms with distinct pigmentation. Body 3,088 (2,061–4,720, n = 25) long, 2,230 (1,277– 3,013, n = 24) wide at level of posterior portion of testis (Fig. 1). Haptor 1,656 (1,142– 2,629, n = 25) in diameter, divided into 1 central and 8 peripheral loculi. Hamuli absent. Fourteen hooklets distributed symmetrically in thicker portion of marginal valve between every 4 papillae (Fig. 1). Haptoral rim with 56 marginal papillae; 8 papillae associated with each posterior loculus; 7 papillae associated with each posterolateral and anterolateral loculus; and 6 papillae associated with each anterior loculus (Fig. 1). Marginal papillae with 6–8 sclerites (Fig. 1); terminal papillar sclerite distinct from other papillar sclerites ( Fig. 2 View FIGURE 2 A). Sclerites on radial, inner and outer ring septa similar ( Fig. 2 View FIGURE 2 B); tripartite sclerites sometimes present at junction of radial septum with inner or outer ring septum ( Fig. 2 View FIGURE 2 C).
Mouth ventral, subterminal. One anterolateral gland duct opening on either side of head (Fig. 1), contains needlelike secretion. Subterminal groove present at anterior end (Fig. 1). Two pairs of eyespots anterodorsal to pharynx; crystalline lenses still present. Pharynx 311 (223–395, n = 25) long, 310 (225–423, n = 25) wide. Intestinal diverticula extend from anterior margin to posterior margin of body proper (Fig. 1). Pigment present, associated with lining of intestinal diverticula, in all specimens collected, including juveniles.
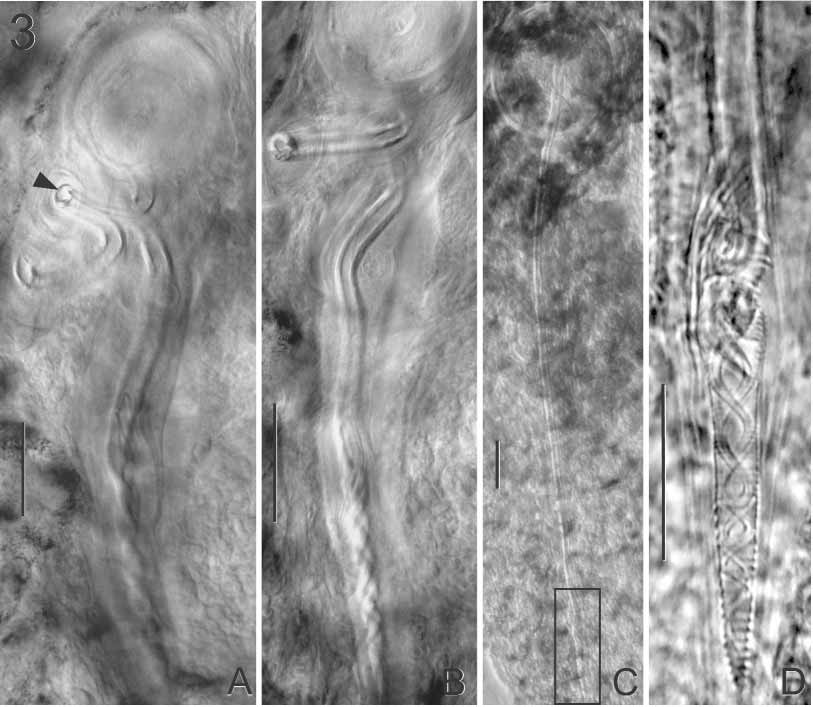
Testis 758 (559–946, n = 17) wide. Vas deferens arises from left side of testis, and runs anteriorly dorsal to vagina, narrows and curves to right side of body ventral to ejaculatory bulb, inflates to form looping seminal vesicle then narrows, entering ejaculatory bulb posteriorly (Fig. 1). Ejaculatory bulb 279 (199–356, n = 25) long, 260 (192–318, n = 25) wide. Male copulatory organ long, sclerotised tube 973 (866–1,105, n = 22) long; distal end distinct with crisscrossed sperm duct and coiled accessory filament ( Figs 2 View FIGURE 2 D, 3D). Muscular sheath surrounds entire length of male copulatory organ (Fig. 1). Proximal end of male copulatory organ looped in juvenile specimens; straightens as worm matures ( Fig. 3 View FIGURE 3 A–C).
Ovary bilobed with one lobe on either side of testis (Fig. 1); fusing medially; single branch loops right intestinal caecum dorsoventrally and forms oviduct. Oviduct receives common vitelline duct and duct from seminal receptacle and forms ovovitelline duct which enters oötype posteriorly. Oötype 330 (180–421, n = 23) long. Mehlis’ gland not seen. Vaginal pore on left side of body at level of anterior portion of ejaculatory bulb (Fig. 1). Proximal part of vagina sclerotised then narrows and enters seminal receptacle. Seminal receptacle 256 (167–410, n = 15) long, 136 (85–200, n = 15) wide (Fig. 1). Sclerotised spermatophore ( Fig. 2 View FIGURE 2 E) present in vagina of 4 specimens; spermatophore 504 (398–614, n = 4) long, bulb diameter 211 (192–229, n = 3).
Dendromonocotyle bradsmithi n. sp. Whole adult parasite, composite drawing, ventral view. NB: For clarity, the intestinal diverticula surrounding and overlying the reproductive organs are not drawn. Abbreviations: alg, anterolateral gland duct opening with needlelike secretion; e, eyespot; eb, ejaculatory bulb; h, hooklet; i, intestinal diverticulum; m, mouth; mco, male copulatory organ; mp, marginal papilla; ms, muscular sheath surrounding male copulatory organ; mv, marginal valve; oot, oötype; o, ovary; p, pharynx; sg, subterminal groove; sr, seminal receptacle; sv, seminal vesicle; t, testis; tps, terminal papillar sclerite; tvd, transverse vitelline duct; v, vagina; vd, vas deferens; vs, sclerotised portion of vagina. Scale bar: 500 m.
Vitellarium inconspicuous, largely obscured by extensive pigmented intestinal diverticula, extends from level of mouth to posterior portion of body proper. Transverse vitelline duct at level of anterior portion of seminal receptacle; common vitelline duct joins oviduct. Egg dimensions below.
Remarks
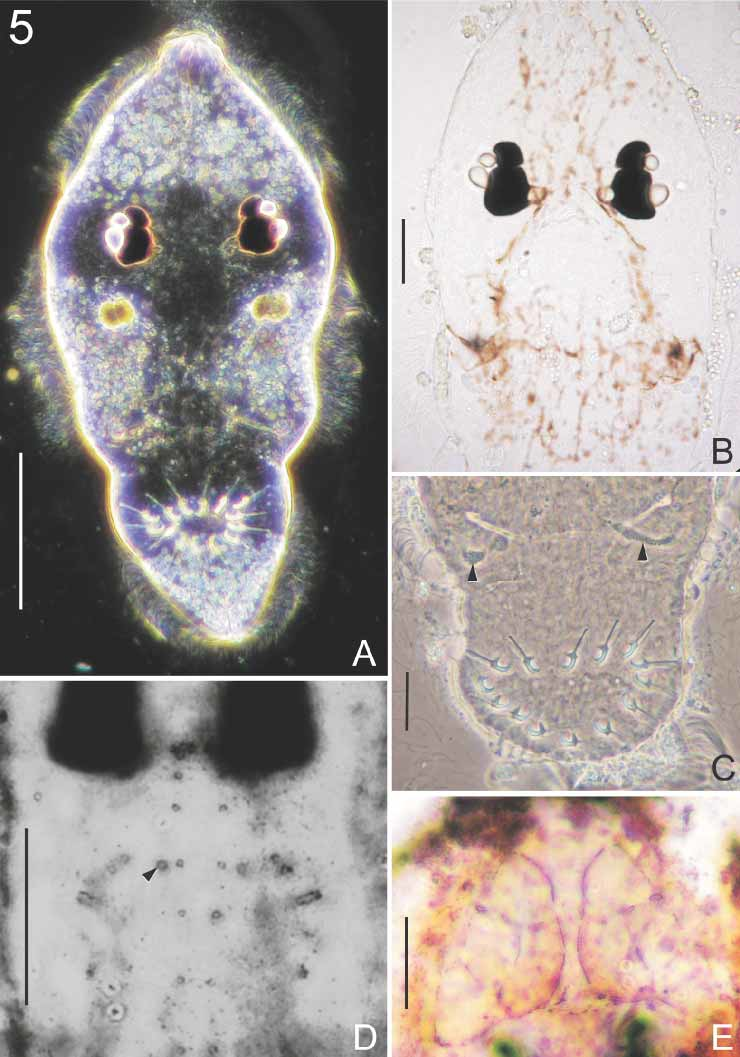
Live specimens attached to the bottom of Petri dishes and exhibited characteristic undulating movements. The undulating movements observed in live D. bradsmithi have also been reported in other skinparasitic monogeneans. Kearn (1962) demonstrated that this characteristic movement in the capsalid Entobdella soleae (van Beneden & Hesse, 1864) Johnston, 1929 increases when oxygen levels in the water decrease and therefore probably aids respiration. Monocotylid larvae usually have a prominent crystalline lens associated with each of the 4 eyespots (e.g. Figs 4 View FIGURE 4 A, 5A). This is the first time the crystalline lenses have been reported to persist in adult monocotylids.
Egg development and hatching
Egg tetrahedral ( Fig. 2 View FIGURE 2 F); side length 113 (106–120, n = 10); measured from freshly laid eggs. Eyespots developed after 5 days and larvae hatched spontaneously after 7 days at 22°C. Batch A: total of 106 eggs, 99 hatched (93% success), 7 embryonated but not hatched. Batch B: total of 411 eggs, 385 hatched (94% success), 21 embryonated but not hatched, 5 unembryonated.
Description of larva ( Figs 4 View FIGURE 4 , 5 View FIGURE 5 A–C)
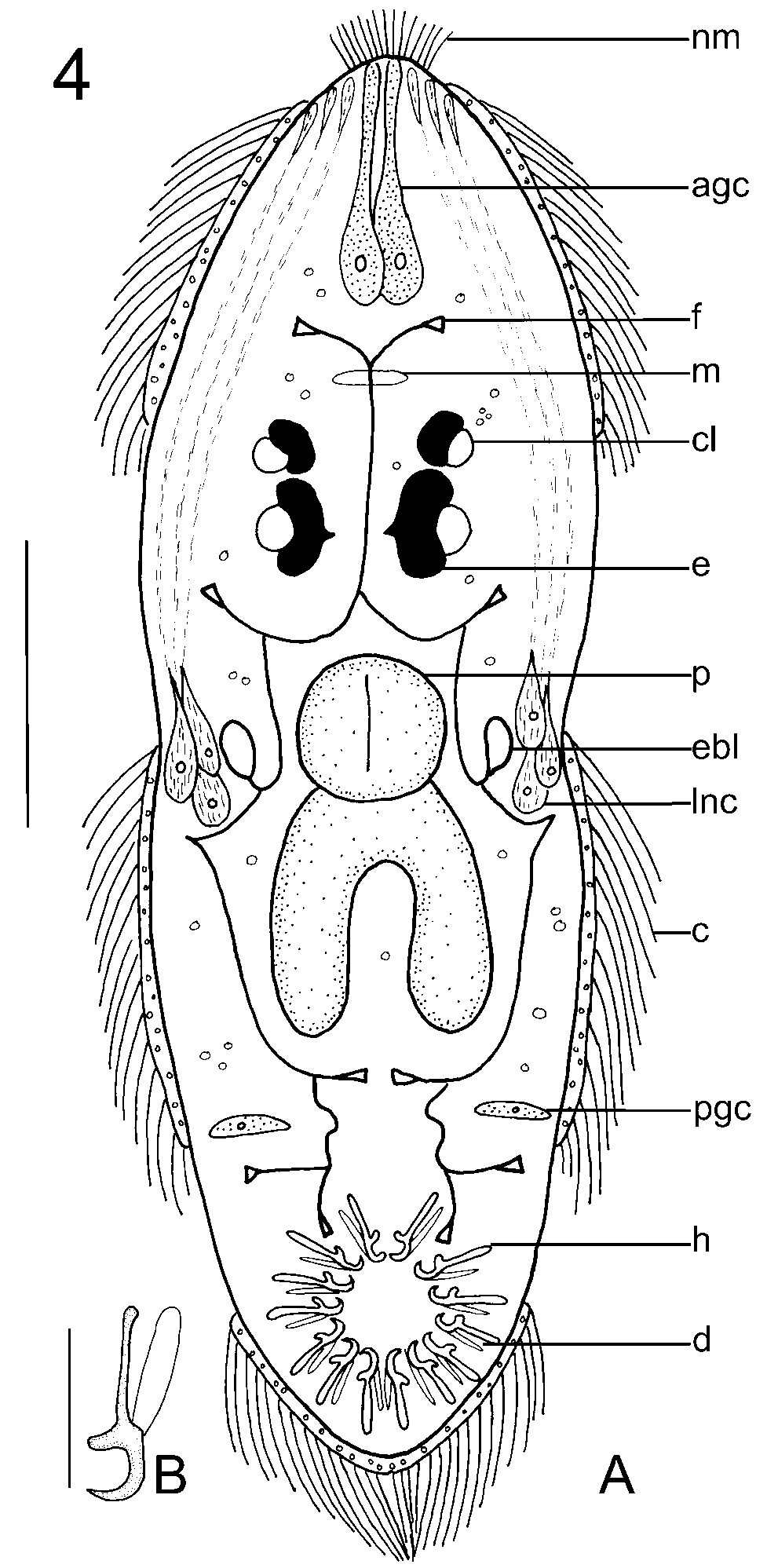
Observations based on 17 living larvae. Larva 187 (168–225, n = 11) long and 74 (60–105, n = 11) wide. Ciliated cells in 3 zones; one anterior band, 2 median patches and ciliated cone on haptor ( Fig. 4 View FIGURE 4 A). Refringent droplets present in epidermal cells bearing cilia ( Figs 4 View FIGURE 4 A, 5A). Group of about 20 nonmotile cilia observed at anterior end of larva. Refringent droplets, probably lipid, observed throughout body ( Fig. 4 View FIGURE 4 A).
Four pigmented eyes with crystalline lenses arranged as illustrated ( Figs 4 View FIGURE 4 A, 5A, 5B). Pigment distributed throughout body of larva ( Fig. 5 View FIGURE 5 B). Pharynx 24 (19–28, n = 11) in diameter. Small bifurcate gut present. Two anteromedian gland cells containing granular secretion; single duct from each gland cell runs anteriorly and opens at anterior margin of head ( Fig. 4 View FIGURE 4 A). Group of 3 gland cells containing needlelike secretion on either side of pharynx ( Fig. 4 View FIGURE 4 A), sometimes obscured by body pigment. One duct from each of these gland cells runs anteriorly and opens at slightly swollen endings on lateral borders of head ( Fig. 4 View FIGURE 4 A). Two posterior gland cells, 1 on each side of body, near haptor containing granular secretion ( Figs 4 View FIGURE 4 A, 5C). Pores or ducts associated with these gland cells not seen.
Five pairs of flame bulbs observed: 1 pair anterior to eyes; 1 pair anterolateral to pharynx; 1 pair posterior to gut; 1 pair in posterior body near posterior gland cells; 1 pair extending into haptor ( Fig. 4 View FIGURE 4 A). Path of ducts connecting flame bulbs as shown ( Fig. 4 View FIGURE 4 A). Excretory bladders distinct, lateral to pharynx.
Haptor with 14 hooklets, each of similar shape and size 14 (13–15, n = 14) long with distinct domus ( Figs 4 View FIGURE 4 A, 4B, 5A, 5C). Hamuli not seen in freshly hatched larvae.
Remarks
In addition to the anteromedian gland cells and the posterior pair of gland cells ( Figs 4 View FIGURE 4 A, 5C), monocotylid larvae described so far generally have 2 other sets of gland cells on either side of the body posterolateral to the pharynx. The first set comprise 3 to 4 gland cells containing needlelike secretion located on either side of the body just posterolateral to the pharynx (see Chisholm & Whittington 1996). The second set comprises a pair of gland cells containing granular secretion located on either side of the body just posterior to the cells containing needlelike secretion (see Chisholm & Whittington 1996). A single duct runs from each of these gland cells and opens at the anterior end of the larva. In contrast to this standard arrangement, we did not detect the pair of lateral granular glands and associated ducts in D. bradsmithi larvae; only 3 gland cells containing needlelike secretion were seen on either side of the pharynx ( Fig. 4 View FIGURE 4 A). Chisholm & Whittington (1995) also only observed 3 gland cells on either side of the body just posterolateral to the pharynx in D. ardea , but these were believed to contain granular secretion. The secretion type in the lateral gland cells should be verified in D. ardea because with this single exception, the arrangement of gland cells is otherwise identical in D. bradsmithi and D. ardea . No gland cells were described or illustrated lateral to the pharynx in D. kuhlii (see Young 1967).
Distribution of oncomiracidial ciliated epidermal cells and sensilla ( Figs 5 View FIGURE 5 D,E, 6)
Thirtythree larvae stained with AgNO3; description based on 18 stained larvae. Anterior zone with 25 ciliated cells distributed over dorsal and ventral surfaces as illustrated ( Fig. 6 View FIGURE 6 ). Two smaller ciliated cells (probably support nonmotile cilia shown in Fig. 4 View FIGURE 4 A) also present at anteriormost tip of larva on dorsal surface ( Fig. 6 View FIGURE 6 A). Small spaces present between some ciliated cells in anterior zone as illustrated ( Figs 5 View FIGURE 5 E, 6A, 6B). Median zone with 11 ciliated cells on either side of larva; most median zone cells located ventrally ( Fig. 6 View FIGURE 6 A, 6B). Spaces not seen between cilated cells of median zone. Haptoral zone with 13 ciliated cells (but see Discussion) ( Fig. 6 View FIGURE 6 A, 6B). No spaces seen between any ciliated cells in haptoral zone. Dorsal and ventral sensilla distributed as shown ( Figs 5 View FIGURE 5 D, 6A, 6B); some sensilla probably obscured by body pigment.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |