Phenakospermum guyannense (Richard) Miquel (1845: 345)
|
publication ID |
https://doi.org/10.11646/phytotaxa.491.3.4 |
|
persistent identifier |
https://treatment.plazi.org/id/03DB0A12-406B-FFCB-E8BA-FCA647523EA9 |
|
treatment provided by |
Marcus |
|
scientific name |
Phenakospermum guyannense (Richard) Miquel (1845: 345) |
| status |
|
Phenakospermum guyannense (Richard) Miquel (1845: 345) View in CoL
≡ Urania guyannensis Richard (1831: 21) .
Type ( lectotype, designated here):— Illustration in L. C. M. Richard & A. Richard (1831: 43–44 Tab. VI). ( Figure 1 View FIGURE 1 )
Figure 2 A–E View FIGURE 2
Arborescent herbs, rhizomatous, aerial shoots 3–8 m tall. Pseudostem palm-like, 2.5–6 m tall, 8–14 cm diam., composed of a central fibrous stem covered with overlapping, sheathing leaf bases. Leaves 7–12, simple, alternate, entire, persisting at the time of flowering, distichous; lamina 1–2.7 × 0.4–0.6 m, venation pinnate, with lateral veins parallel to each other, base cordate, apex obtuse, adaxial surface dark green, abaxial surface glaucous; petiole green, ± glaucous, 100–120 cm long, 2.5–3.7 cm diam. Inflorescence terminal, erect, up to 3 m tall, held conspicuously above the leaves, consisting of a peduncle, rachis, and bracts; peduncle green, glaucous, 1.2–1.5 m long, 7–18 cm diam.; rachis 45–90 cm long, 2.5–9 cm diam., internodes 6–24 cm long; bracts persistent, coriaceous becoming papery at fruit maturity, boat-shaped, distichous, 3–8 per inflorescence, oriented 45–90 degrees to the axis of the inflorescence, green to yellowish, glaucous, with mucilaginous secretions, 30–45 × 5–12 cm, each subtending a cincinnus of up to 25 flowers. Flower dichlamydeous, homochlamydeous, hermaphroditic, cream-colored to white; pedicel 1–1.2 cm long, cream-colored; sepals and petals, acuminate, glabrous; dorsal sepal creamy white, 12–15 × 1.5–2 cm, slightly imbricate, two lateral sepals creamy white, 10–12 × 1.5–2 cm; two lateral petals creamy white, connate, overlapping but free at base, fused from middle to apex, margins becoming revolute, 10–11 × 1.5–1.8 cm, and one free dorsal petal, 10–11 cm × 1.5–1.8 cm, creamy white with thin, dark green margins; stamens five, filaments 10–11 cm long, linear, geniculate, attached basally to the apex of the perianth tube and distally to the apex of the fused petals; anthers linear, ca. 7 cm long, dehiscence longitudinal; ovary inferior, ca. 3 × 2 cm, bicarinate, white, three-celled, ovules numerous in 4 rows per locule, straight, lax, style filiform, ca. 15 cm long, stigma ovate, ca. 2.0 cm long. Fruit capsular, ovate, loculicidal, woody, 10–13 × 4.5–6 cm. Seeds arillate, aril composed of bright red-orange threads, aromatic, 0.7–1 × 0.6–0.8 cm.
Nomenclatural notes and typification:— We observed that the authorship for Urania guyannensis varied depending on the plant name index consulted, attributing the species to L.C.M. Richard (e.g. IPNI 2020, Flora do Brasil 2020, Tropicos 2020) or A. Richard (e.g. Bernal et al. 2019, The Plant List 2020, WCSP 2020). After L.C.M. Richard’s death in 1821, Achille Richard, his son, published some studies conducted by his father as in memoriam. One of these entitled “ De Musaceis commentatio botanica, sistens characteres hujus-ce familiae generum’’ was published in 1831 and presents the original description of Urania guyannense and some other species of Musaceae . In the first pages of the work, A. Richard states that “ Dans ce Mémoire, comme dans les deux autres de mon père, que j’ai publiés après sa mort, les descriptions seules ont été faites par lui; toutes les généralités qui précèdent, c’est-à-dire la description générale de la famille, les rapports des Musacées avec les autres plantes monocotylédones, enfin, les caractères généraux de la famille et des genres qui la composent, ont été rédigés par moi.” (L.C.M. Richard & A. Richard 1831:1) meaning that the descriptions were provided by his father L.C.M. Richard, while information about the relations of the genera of the family and the classification of Musaceae amongst other Monocots was made by himself. We understand that, according to article 46.2 of the ICNAFP ( Turland et al. 2018), this statement clearly defines L.C.M. Richard as the sole author of the species described in this work. Other species described in the same work, also present A. Richard as the species author in some plant name indexes, a mistake that should be corrected.
The description of Urania guyannensis cites no specimens in the protologue other than two illustrations (plates VI and VII). The description is based on a fruiting individual as is presented in the original description “ Flores non vidi. Color, si qua fides incolis, luteus ” meaning that no flowers were used. The herbaria P and G, where Richard’s collections are stored ( Stafleu & Cowan 1983), were examined and curators were consulted in order to search for any fruiting specimen collected before 1821, that could be linked to the original material, but no specimen was found. In view of the above, one of the illustrations presented on Richard’s work should be designated as the lectotype. Here we chose plate VI, as it presents a clear image of the plant habit and inflorescence which permits its recognition among other Strelitziaceae . Later, Endlicher (1833) wrote about this species as a distineti generis ( Phenakospermum dicendi ), he only created the genus Phenakospermum but did not combine the species. Miquel (1845) cited the work of Endlicher and combined the species, including Phenakospermum guyannense .
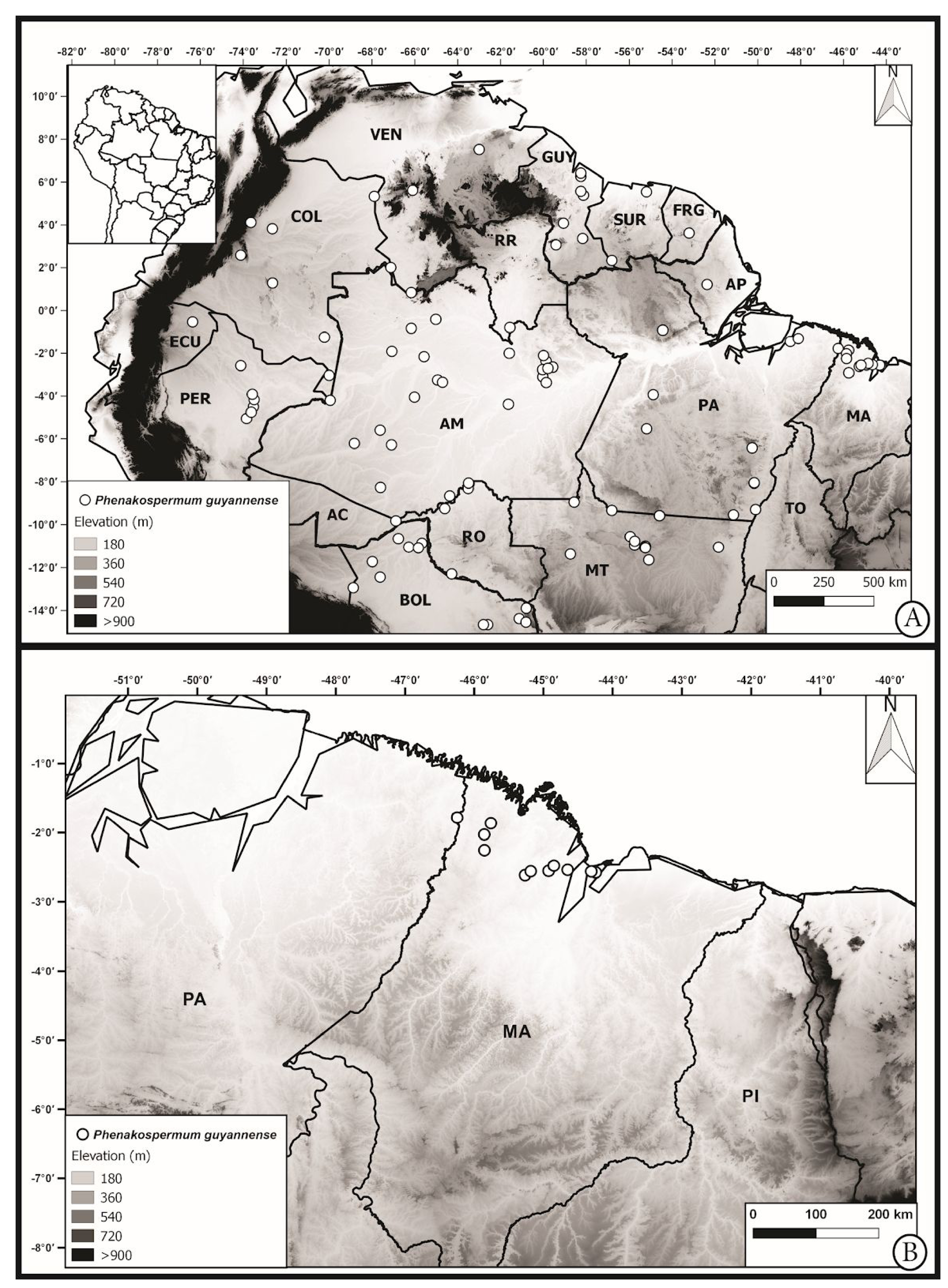
Notes on Distribution: — Phenakospermum guyannense is endemic to South America ( Funk et al. 2007). In Brazil, it is reported for the following regions: North ( Acre, Amazonas, Amapá, Pará and Rondônia states), Centralwest ( Mato Grosso state) and Northeast ( Maranhão state) ( Almeida & Antar 2020).
In Maranhão, Phenakospermum guyannense was found in 10 municipalities located in the Amazon region ( Figure 3 View FIGURE 3 ). These are the first records in those municipalities. Previously to these new collections, the precise occurrence of P. guyannense in Maranhão state was inaccurate. The only known past collection for the State with coordinates lacks reproductive structures, making it difficult to precise its identification, while the other collection, made more than 50 years ago, indicated the locality as only Babacal municipality. Besides, the low level of collection of this giant herb ( Womersley 1981; Kress & Stone 1993) makes the distribution and conservation analysis problematic. In view of that, new efforts to collect Phenakospermum through the Amazonia domain are still necessary. For example, the Brazilian states of Tocantins and Roraima also have areas inserted in the Amazonia domain and further collections may uncover new populations of P. guyannense in these states.
Popularly known as sororoca, this species was collected in Maranhão state in very sunny places such as roadside marshes, but some individuals were also collected in shady areas. It was observed that populations generally tend to form large clusters, an ecological feature that Perigolo et al. (2017) used to define a vegetation subtype in their study area, the open ombrophilous forest with sororoca. This vegetation subtype is one of the most frequent forest formations in the upper Madeira River in Rondônia, Brazil ( Perigolo et al. 2017). Meanwhile, P. guyannense is among the ten most abundant species of upland Bolivian Amazon forest, with 22 individuals per hectare ( Araujo-Murakami et al. 2015).
Furthermore, the disjunct populations of this species suggested that plants of coastal environments dispersed along the margin of this ‘mega-wetland’ ( Hoorn et al. 2010) and reinforced the influence of marine interference on the most species-rich ecosystems ( Antonelli et al. 2018).
New Records collected in Maranhão: BRAZIL. Maranhão : Alcântara, Brejo ensolarado na MA-106. 02°32’24”S, 44°39’05”W, 28 October 2019, W. R GoogleMaps . Silva Junior & A. W. C . Ferreira 316 ( SLUI 5613 ). Bequimão, fragmento de mata na MA-106. 02°28’43”S, 44°51’01”W, 28 October 2019, W. R GoogleMaps . Silva Junior & A. W. C . Ferreira 317 ( SLUI 5614 ). Boa Vista do Gurupi, Margem da BR-31. 01°46’52”S, 46°14’47”W, 04 March 2019, W. R GoogleMaps . Silva Junior & A. W. C . Ferreira 314 ( SLUI 5616 ). Cândido Mendes, Fazenda Sete Irmãos. 01°51’43”S, 45°45’37” W; 26 October 2019, W. R GoogleMaps . Silva Junior & A. W. C . Ferreira 313 ( SLUI 5617 ). Governador Nunes Freire, estrada para a Fazenda Sete Irmãos. 02°01’36”S, 45°51’23 W, 26 October 2019, W. R GoogleMaps . Silva Junior & A. W. C . Ferreira 319 ( SLUI 5620 ). Maranhãozinho, Margem da BR-316. 02°15’14”S, 45°51’14”W, 26 October 2019, W. R GoogleMaps . Silva Junior & A. W. C . Ferreira 320 ( SLUI 5618 ). Presidente Sarney, estrada para a MA-337. 02°36’59”S, 45°16’01”W; 25 October 2019, W. R GoogleMaps . Silva Junior & A. W. C . Ferreira 312 ( SLUI 5619 ). Peri Mirim, Brejo ao lado da MA-106. 02°33’17”S, 44°55’31”W, 28 October 2019, W. R GoogleMaps . Silva Junior & A. W. C . Ferreira 315 ( SLUI 5621 ). Pinheiro, fragmento de mata próximo a um açude na MA- 337. 02°33’37”S, 45°11’03”W, 25 October 2019; W. R GoogleMaps . Silva Junior & A. W. C . Ferreira 318 ( SLUI 5622 ). São Luís, Universidade Federal do Maranhão. 02°33’47”S, 44°18’35” W, 08 June 2018, W. R GoogleMaps . Silva Junior & A. W. C . Ferreira 311 ( SLUI 5615 ). Parque Estadual do Bacanga. 02°33’41”S, 44°14’43’’W, 08 June 2018, W. R GoogleMaps Silva Junior & A. W. C . Ferreira 310 ( SPF 245500 About SPF ) .
Additional material in Maranhão: BRAZIL. Maranhão : Bacabal, 27 June 1972, D. Sucre & J. F . da Silva 9392 ( RB00630514 , RBCarpo 4629-00948228). Monção, P. I . Guajá , Rio Turiaçu. Guajá Indians. 3°07’S, 46°4’W, 17 June 1987, W. L GoogleMaps . Balée 3350 ( NY00910071 ) .
Preliminary Conservation Status: —The Area of Occupancy is 225,000 km ² and the Extent of Occurrence is 5,759,117.931 km ². Some of the collections were found inside protected areas, for example: the Parque Nacional do Juruema in Amazonas state and the Parque Nacional Montanhas do Tucumaque along the frontier of Brazil and French Guiana. The conservation status of this species is preliminarily assessed as Least Concern ( IUCN 2012). Still, in view of the recent fires and increased deforestation in the Amazon domain ( Stropp et al. 2020), some populations of the species may be locally extirpated, jeopardizing the long-term conservation of the species. This is especially true for the population in Maranhão state, the most threatened area of the Brazilian Amazon ( Almeida & Vieira 2010, Martins & Oliveira 2011, Celentano et al. 2017, Celentano et al. 2018, Silva Junior et al. 2020).
| L |
Nationaal Herbarium Nederland, Leiden University branch |
| C |
University of Copenhagen |
| M |
Botanische Staatssammlung München |
| A |
Harvard University - Arnold Arboretum |
| VI |
Mykotektet, National Veterinary Institute |
| W |
Naturhistorisches Museum Wien |
| R |
Departamento de Geologia, Universidad de Chile |
| J |
University of the Witwatersrand |
| F |
Field Museum of Natural History, Botany Department |
| P |
Museum National d' Histoire Naturelle, Paris (MNHN) - Vascular Plants |
| I |
"Alexandru Ioan Cuza" University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Phenakospermum guyannense (Richard) Miquel (1845: 345)
| Almeida, Roberto Baptista Pereira, Antar, Guilherme Medeiros, Ferreira, Alessandro Wagner Coelho, Junior, Wagner Ribeiro Da Silva, Oliveira, Miguel Sena De & Saraiva, Raysa Valéria Carvalho 2021 |
Phenakospermum guyannense (Richard)
| Miquel, F. A. W. 1845: ) |
Urania guyannensis
| Richard, L. C. & Richard, A. 1831: ) |