Erythrolamprus macrosomus ( Amaral 1935 ) Ascenso & Costa & Prudente, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4586.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:7BBCFF79-DE38-4A79-8905-7840A1C1955F |
|
persistent identifier |
https://treatment.plazi.org/id/03DC1517-FF84-FF8F-C7FD-2A0BFD38FAC9 |
|
treatment provided by |
Plazi |
|
scientific name |
Erythrolamprus macrosomus ( Amaral 1935 ) |
| status |
stat. nov. |
Erythrolamprus macrosomus ( Amaral 1935) New Status
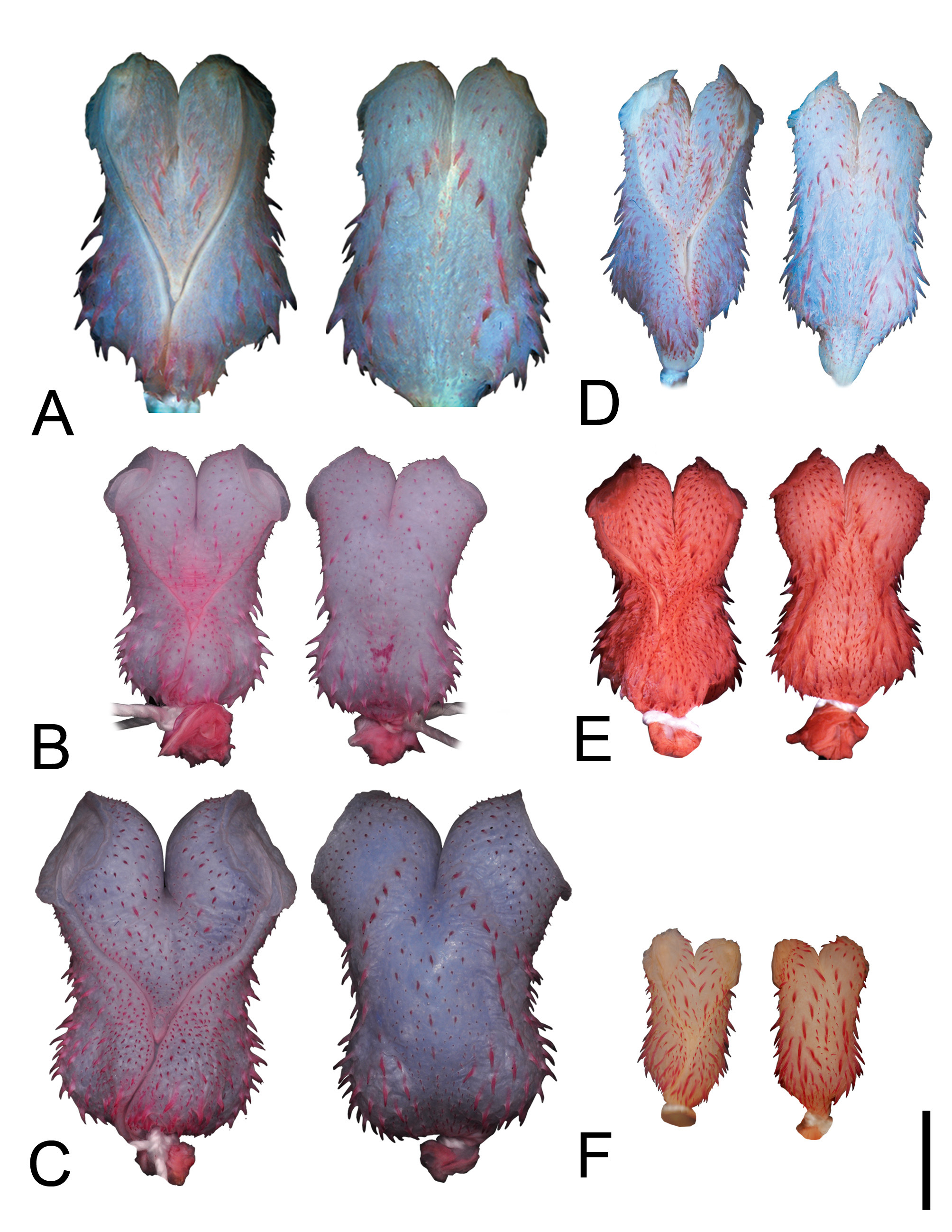
Figures 2C View FIGURE 2 , 3C View FIGURE 3 , 4A View FIGURE 4 , 6C View FIGURE 6
Leimadophis reginae macrosoma Amaral 1935 ; Mem. Inst. Butantan:238. Terra typica: Cana Brava, Minaçu , Goiás, Brazil.
Syntypes: IBSP 9130–33. Leimadophis reginae maculicauda Hoge 1952 ; Mem. Inst. Butantan:241. Terra typica: Sarandi , São Paulo , Brazil. Holotype:
IBSP 9606, lost at the fire accident on the Instituto Butantan. Leimadophis regina macrosoma — Hoge 1958; Mem. Inst. Butantan:69. Liophis reginae macrostoma — Dixon 1980; Milwaukee Publ. Mus .31:24. Liophis reginae macrosomus — Dixon 1983a; Ann. Carnegie. Mus . 52:3. Erythrolamprus reginae macrosomus — Grazziotin et al. 2012; Cladistics. 1:21. Liophis reginae — Wallach et al. 2014; Snakes of the World:393.
Syntypes. Four specimens, IBSP 9130–33 View Materials , collected at Cana Brava , Minaçu , Goiás, Brazil. All type were lost at the fire accident in the Instituto Butantan .
Diagnosis. Erythrolamprus macrosomus is distinguished from all congeners by unique combination of the following characters: (1) dorsal scale rows 17, reducing to 15 rows after midbody; (2) apical pit single; (3) ventrals 137–169 in females and 140–158 in males; (4) subcaudals 53–91 in females and 67–87 in males; (5) dorsum of head olive green, extending to anterior third of the body, gradually changing to grayish-brown at midbody; (6) upper edges of supralabials with the same color of dorsum of head; (7) belly creamish-white with black spots with squared or rhomboid shape arranged in a checkered pattern; (8) lateral black spots absent; (9) ventral surface of tail creamish-white with dots or spots near the cloacal shield; (10) intrasulcal region of hemipenial body with spinules homogeneously distributed; (11) medial region of hemipenial body at asulcate face ornamented with two rows of elongated spines; (12) sulcus spermaticus bifurcates at the half length of hemipenial body; and (13) moderate body size (SVL 136–698 mm).
Comparisons. Erythrolamprus macrosomus shares a lateral stripe along the posterior region of the body and the tail, and usually a cream belly with black spots of square or rhomboid shape with E. reginae , E. dorsocorallinus , E. zweifeli and, eventually E. oligolepi s regarding ventral marks. Erythrolamprus macrosomus differs from E. oligolepis by having 17 dorsal scale rows at midbody and conspicuous ventral rectangular dark spots (vs. 15 dorsal scales rows at midbody and belly usually without spots); from E. reginae by having tail cream with black spots (vs. tail cream without spots); from E. zweifeli and E. dorsocorallinus by having dorsal ground color dark brown (vs. dorsal scales with apical half black and the basal portion yellowish, reddish or bluish-cream); from E. cobella , E. taeniogaster and E. breviceps by having belly cream with spots occupying an area lower than a ventral scale (vs. cream belly scattered with complete black bands, usually occupying two ventral scales). Additionally, E. macrosomus differs from E. e. epinephelus and E. e. a lbiventris by having a ventral surface with squared black spots (vs. belly usually without spots); from E. e. juvenalis, E. e. pseudocobella, E. e. opisthotaenius, E. e. bimaculatus, E. e. lamonae, and E. e. fraseri by having dorsal ground color of head regularly pigmented of olive green, extending to anterior third of the body, without bands or blotches (vs. dorsum of the head cream or olive green with bands on anterior portion of body, and a black spot and a thick postorbital stripe on each side of head); from from E. taeniurus by having dorsal ground color uniformly olive green and 141–169 ventrals (vs. dorsum with black bands and 152–181 ventrals). Regarding the sympatric taxa, E. macrosomus differs from E. miliaris by having dorsal ground color uniformly olive green with a dorsal-lateral black stripe (vs. dorsum of head, body and tail yellowish-cream with the distal half of the scales colored black and dorsal-lateral stripe absent); from E. typhlus and E. poecilogyrus by having 17 dorsal scale rows in the midbody (vs. 19 dorsal scale rows in the midbody).
Description of a topotype. Adult female, MCP 13063 ( Fig. 4A View FIGURE 4 ); Body cylindrical; SVL 557 mm, CL 196 mm (35.2% SVL); head length 22.2 mm, height 7.8 mm, and width 11.3 mm; diameter of ocular orbit 3.9 mm; distance between orbit and rostral shield 5.2 mm; rostral triangular, 4.0 mm wide and 2.8 mm high, visible in dorsal view; internasals two, 1.9 mm length, and 1.7 mm wide; prefrontals two, 2.7 mm length, and 2.7 mm wide, in contact with supraoculars, preocular, loreal, and postnasal; frontal pentagonal, 5.6 mm length, and 3.8 mm wide; parietals two, 6.8 mm length, and 4.1 mm wide; supralabials eight, second and third contacting loreal, fourth to fifth contacting eye, and sixth and seventh higher than the remaining supralabials; supraoculars longer than wide; nasal in contact with first two supralabials, internasals, prefrontals, loreal, and rostral; loreal tetragonal, 1.4 mm length and 2.1 mm high, contacting second and third supralabials, postnasal, prefrontals, and preocular; preocular contacting supraocular, prefrontal, nasal, third and fourth supralabials; postoculars two, upper postocular higher than lower; temporals 1+2, anterior longer than upper posterior temporal; symphysial triangular; infralabials 9, first pair in broad contact behind symphysial and first four pairs contacting chinshields; anterior chinshields 5.5 mm long; posterior chinshields 5.9 mm long; smooth dorsal scale rows 17/17/15, reduction at the level of 85/85 th ventrals (right/left); apical pit single; ventrals 153; subcaudals 81; cloacal plate divided.
Dorsum of head dark brown, darker than body; upper edges of supralabials dark brown; gular region and supralabials creamish-white; dorsal ground color of body gray to greyish-brown on anterior portion, without spots; dorsal-lateral stripe barely distinctive, starting at middle or at posterior third of body extending to distal portion of the tail; dorsal stripe dark brown, occupying vertebral and two paravertebral rows, on each side of body, extending from posterior third of body to the end of tail; ventral region of body creamish-white with rectangular spots; tail creamish-white with spots or dots usually more concentrated on the first rows of subcaudals.
Morphometric and meristic variation (n= 164). SVL 136–698 mm (mean= 419.3; SD= 115.8; n= 141) and CL 44–262 mm (mean= 152.2; SD= 50.4; n= 117); head length 10.4–29.7 mm (mean= 20.2; SD= 3.6; n= 125), height 4.0– 10.4 mm (mean= 7.4; SD= 1.3; n= 121), and width 5.2–15.3 mm (mean= 10.6; SD= 1.9; n= 120); diameter of ocular orbit 1.9–5.2 mm (mean= 3.7; SD= 0.6; n= 125); distance between orbit and rostral shield 2.1– 7.4 mm (mean= 4.9; SD= 0.9; n= 125); rostral triangular, 1.5–7.1 mm wide (mean= 3.7; SD= 0.7; n= 124), 1.2–5.7 mm high (mean= 2.4; SD= 0.5; n= 124); internasals 0.7–6.7 mm long (mean= 1.8; SD= 0.6; n= 125), 1.0– 5.2 mm wide (mean= 2.4; SD= 0.6; n= 122); prefrontals, 1.2–5.0 mm long (mean= 2.5; SD= 0.5; n= 125), 1.6–6.9 mm wide (mean= 3.2; SD= 1.2; n= 125); frontal pentagonal, 3.4–11.3 mm long (mean= 5.5; SD= 0.9; n= 125), and 1.8–4.4 mm wide (mean= 3.3; SD= 0.5; n= 102); parietals 2.6–8.3 mm long (mean= 6.4; SD= 1; n= 101), 2.0– 5.3 mm wide (mean= 3.7; SD= 0.6; n= 102); loreal tetragonal, 0.6–5.9 mm long (mean= 1.3; SD= 0.5; n= 102), 0.7–4.1 mm high (mean= 1.8; SD= 0.4; n= 102); infralabials 8–11 (mean= 9.9; SD= 0.3; n= 158); infralabials contacting chinshileds 3–6 (mean= 4.9; SD= 0.3; n= 163); anterior chinshields 2.5–6.5 mm long (mean= 4.8; SD= 0.8; n= 125), posterior chinshields 1.4–8.5 mm long (mean= 5.4; SD= 1.1; n= 126); dorsal scale rows 17 with reduction at the level of 67– 95/70–96 ventrals (right/left; n= 121); ventrals 137–169 in females (mean= 151.4; SD= 5.3; n= 70), 140–158 in males (mean= 151.3; SD= 4.1; n= 70); subcaudals 53–91 in females (mean= 76.6; SD= 7.7; n= 54), 67–87 in males (mean= 78.3; SD= 4.9; n= 52).
Color pattern in preservative. Dorsum of head dark brown, darker than body; upper edges of supralabials with indistinct postorbital stripe; gular region and supralabials creamish with two or three marks on the first three infralabials; dorsal ground color on anterior third of body gray to greyish-brown, without spots; barely defined dorsal-lateral stripe starting on the posterior third of body and extending to distal portion of tail; dorsal stripe dark brown, occupying vertebral and two paravertebral rows on both sides of body, extending from posterior third of body to end of tail; ventral region of body cream with rectangular spots; tail creamish-white with spots and points usually more concentrated on the first rows of subcaudals ( Fig. 2C View FIGURE 2 ).
Ontogenetic variation of color in preservative. Hatchlings and juveniles with dorsum of head uniformly green without nuchal collar; belly creamish-white little spotted; ventral surface of tail without black spots or dots.
Color in life. Dorsum of head dark brown gradually changing to olive green until midbody; supralabials and gular region white; first two dorsal scale rows grey to cream ( Fig. 6C View FIGURE 6 ).
Hemipenial morphology (everted organ n= 4). Fully everted and maximally expanded hemipenes renders slightly bilobed, non-calyculate, and non-capitate organs; hemipenis short with smooth apical disk at surface of lobes; hemipenial body covered by spinules, more concentrated on the marginal region of sulcus spermaticus; lobes with spinules concentrated over lateral region on both sides of organ; sulcus spermaticus bifurcates at the half length of hemipenial body; branches with centrifugal orientation extending to central region of apical disks; basal region of sulcus with an inflated area ornamented with elongated spines; intrasulcal region with spinules homogeneously distributed; proximal region of hemipenial body at asulcate face with bulges on either side, ornamented with elongated spines; medial region of hemipenial body at asulcate face ornamented with two rows of elongated spines; region around intrasulcal spines with an area without ornamentation ( Fig. 3C View FIGURE 3 ).
Geographic distribution. Erythrolamprus macrosomus occurs in semidecidual forests of the Atlantic Forest in Paraná and São Paulo states, in the Brazilian Cerrado in Goiás, Mato Grosso, Mato Grosso do Sul, Minas Gerais, São Paulo , and Paraná states, and in the Chacos from Paraguay and Angertina ( Giraudo 2001). The range of distribution was recently extended to El Palmar National Park, province of Entre Ríos, Argentina, representing the southernmost record ( Arzamendia 2016) ( Fig. 8 View FIGURE 8 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Erythrolamprus macrosomus ( Amaral 1935 )
| Ascenso, Alexandre C., Costa, João C. L. & Prudente, Ana L. C. 2019 |
Leimadophis reginae maculicauda
| Hoge 1952 |
Leimadophis reginae macrosoma
| Amaral 1935 |