Erythrolamprus oligolepis ( Boulenger 1905 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4586.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:7BBCFF79-DE38-4A79-8905-7840A1C1955F |
|
persistent identifier |
https://treatment.plazi.org/id/03DC1517-FF86-FF81-C7FD-29DBFB01FB02 |
|
treatment provided by |
Plazi |
|
scientific name |
Erythrolamprus oligolepis ( Boulenger 1905 ) |
| status |
|
Erythrolamprus oligolepis ( Boulenger 1905)
Figures 1E View FIGURE 1 , 2B View FIGURE 2 , 3B View FIGURE 3 , 6B View FIGURE 6
Liophis oligolepis Boulenger 1905 ; Ann. Mag. Nat. Hist. 15:455. Terra typica: “Igarape assu”. Holotype: BMNH 1946.1.4.66. Leimadophis oligolepis — Gomes 1918; Mem. Inst. Butantan:58.
Liophis reginae semilineata — Dixon 1983a; Ann. Carnegie. Mus View in CoL . 52:3 (part.).
Liophis oligolepis — Cunha & Nascimento 1993; Ofídios da Amazônia:73.
Erythrolamprus oligolepis — Grazziotin et al. 2012; Cladistics. 1:21.
Liophis oligolepis — Wallach et al. 2014; Snakes of the world:391.
Holotype: Adult male, BMNH 1946.1 .4.66, collected by M.A. Robert at municipality of “Igarape Assu” (rectified to Igarapé-Açu by Cunha and Nascimento 1993), state of Pará, Brazil (specimen photographs examined, Fig. 1E View FIGURE 1 ).
Diagnosis. Erythrolamprus oligolepis can be distinguished from all congeners by unique combination of the following characters: (1) dorsal scales rows 15, without reduction at the midbody; (2) apical pit single; (3) ventrals 134–166 in females and 142–160 in males; (4) subcaudals 55–67 in females and 53–64 in males; (5) dorsal coloration of the head olive green, extending to anterior third of the body, gradually changing to grayish-brown at midbody; (6) upper edges of supralabials with distinctive dark postorbital stripe; (7) belly creamish-white usually without black spots, except for the specimens of the west margin of the Amazon River, which present a slight pigmentation; (8) lateral black spots extending from anterior third of the body, between 2–3 th dorsal scale rows, to form a lateral stripe, which extends to the end of the tail; (9) subcaudals without dots or spots; (10) intrasulcal region of hemipenial body with spines slightly elongated, arranged in a row extending from distal region of lobes to the level of bifurcation of sulcus spermaticus; (11) medial region of asulcate face of hemipenial body ornamented with spinules homegeneously distributed; (12) sulcus spermaticus bifurcates at half length of the hemipenial body; and (13) small body size (SVL 114–392 mm).
Comparisons. Erythrolamprus oligolepis shares a lateral stripe along the posterior region of the body and the tail, and usually a cream belly with black spots of square or rhomboid shape with E. reginae , E. macrosomus , E. dorsocorallinus , and E. zweifeli . Erythrolamprus oligolepis differs from reginae and E. macrosomus by having 15 dorsal scale rows at midbody and belly usually without spots (vs. 17 dorsal scales rows at midbody and ventral rectangular dark spots); differs from E. zweifeli and E. dorsocorallinus by having dorsal ground color regularly pigmented of dark brown (vs. dorsal scales with apical half black and the basal portion yellowish-cream, reddish or bluish-cream). Additionally, E. oligolepis differ from all subspecies of E. epinephelus by having dorsal ground color of head regularly pigmented of olive green, extending from anterior third of the body, without bands or blotches, 15 dorsal scale rows at midbody, and belly usually without spots (vs. dorsum of the head cream or olive green, with bands on anterior portion of body and with a black spot and a thick postorbital stripe on each side of head, 17 dorsal scales rows at midbody, and belly with dark rectangular spots); differs from E. pyburni by having the anterior third of the body without spots, subcaudals 55–67 in females, and 53–64 in males, fourth and fifth supralabials in contact with the eye, and nine infralabial scales (vs. anterior third of the body with transversal spots, 37–40 subcaudals, third and fourth supralabials in contact with the eye, and eight infralabial scales).
Redescription of the holotype. Body cylindrical; SVL 247 mm, CL 73 mm (29.6% SVL); internasals two, barely wider than long; prefrontals two, twice longer than wide, in contact with supraoculars, preocular, loreal and postnasal; frontal pentagonal; parietals two; supralabials eight, fourth to fifth contacting eye; supraoculars longer than wide; nasal in contact with first two supralabials, internasals, prefrontals, loreal, and rostral; loreal tetragonal, higher than long, contacting second and third supralabials, postnasal, prefrontals, and preocular; rostral barely triangular, wider than high, visible in dorsal view; preocular contacting supraocular, prefrontal, nasal, third and fourth supralabials; postoculars two, upper postocular higher than lower; temporals 1+2, anterior longer than upper posterior temporal; symphysial triangular; infralabials 9, first pair in broad contact behind symphysial; first four pairs in contact with chinshields; smooth dorsal scale rows 15/15/15, without reduction; apical pit single; ventrals 152; subcaudals 61; cloacal plate divided.
Dorsum of head dark gray to dark brown; upper edges of supralabials with a distinctive postorbital stripe; lateral black spots extending from anterior third of the body, located between 2–3 th dorsal scale rows, and merged on the level of cloacal plate to form a lateral stripe, which extends to the end of the tail; ventral surface of body and tail creamish without dots or spots.
Morphometric and meristic variation (n= 84). SVL 114–392 mm (mean= 294.1; SD= 70; n= 84) and CL 31–136 mm (mean= 92.3; SD= 23.8; n= 83); head length 6.5–19.5 mm (mean= 13.2; SD= 2.2; n= 80), height 3.3– 8.5 mm (mean= 5.2; SD= 1.1; n= 78), and width 4.6–9.6 mm (mean= 7.0; SD= 1.2; n= 78); diameter of ocular orbit 1.3–3.8 mm (mean= 2.8; SD= 0.4; n= 79); distance between orbit and rostral shield 2.1–4.2 mm (mean= 3.2; SD= 0.5; n= 79); rostral triangular, 1.7–3.4 mm wide (mean= 2.7; SD= 0.4; n= 78), 1.0– 2.9 mm high (mean= 1.8; SD= 0.3; n= 78); two internasals, 0.7–1.9 mm long (mean= 1.2; SD= 0.3; n= 79), 1.0– 2.1 mm wide (mean= 1.4; SD= 0.3; n= 79); prefrontals 1–3 mm long (mean= 1.6; SD= 0.3; n= 80), 1.0– 2.6 mm wide (mean= 1.9; SD= 0.3; n= 80); frontal pentagonal, 2.9–5.0 mm long (mean= 4; SD= 0.5; n= 80), 1.5–3.2 mm wide (mean= 2.4; SD= 0.4; n= 80); parietals two, 2.6–6.1 mm long (mean= 4.5; SD= 0.7; n= 80), 1.8–4.8 mm wide (mean= 2.8; SD= 0.4; n= 79); loreal 0.4–1.2 mm long (mean= 0.8; SD= 0.2; n= 80), 0.5–1.8 mm high (mean= 1.2; SD= 0.3; n= 79); infralabials 8–10 (mean= 9.1; SD= 0.6; n= 82); infralabials contacting chinshileds 4–5 (mean= 4.3; SD= 0.5; n= 84); anterior chinshields 2.0– 4.5 mm long (mean= 3.3; SD= 0.6; n= 80), posterior chinshields 2.0– 4.7 mm long (mean= 3.3; SD= 0.7; n= 80); dorsal scale rows 15, without reduction; ventrals 134–166 (mean= 147.1; SD= 6.1; n= 53) in females, 142–160 in males (mean= 149.2; SD= 3.6; n= 31); subcaudals 55–67 in females (mean= 61.3; SD= 3.2; n= 49), and 53–64 in males (mean= 60.2; SD= 2.7; n= 29).
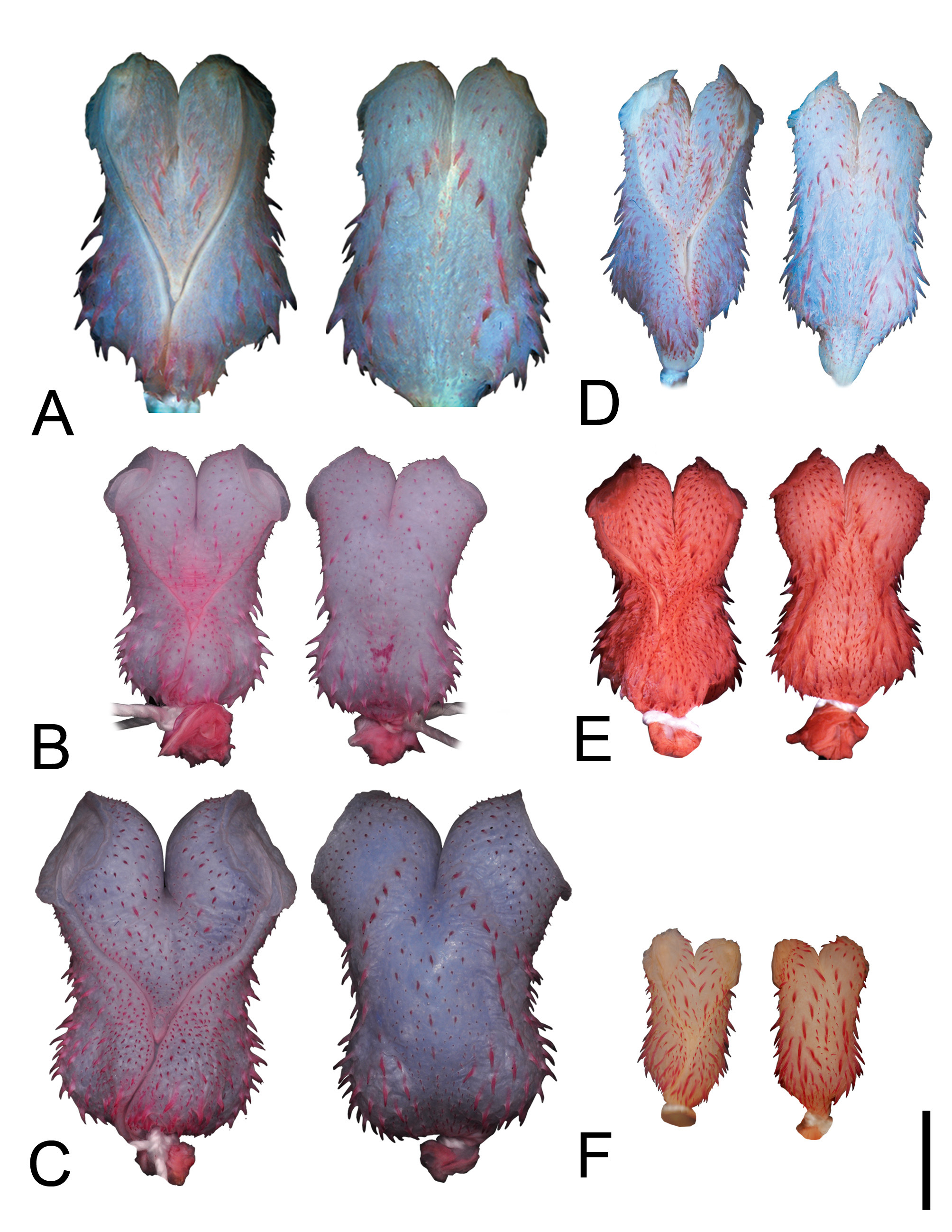
Color pattern in preservative. Dorsum of head dark brown, darker than body; upper edges of supralabials with distinctive postorbital stripe; gular region and supralabials creamish white; nuchal collar cream, three dorsal scales long, anterior to first lateral spot; dorsal ground color on anterior third of body gray to greyish-brown, without spots; dorsal stripe dark brown, occupying vertebral and two paravertebral rows on each side of the body, extending from posterior third of body to end of tail; ventral region of body and tail cream without dots or spots; lateral black spots extending from anterior third of the body, between 2–3 th dorsal scale rows, to form a lateral stripe, which extends to the end of the tail ( Fig. 2B View FIGURE 2 ).
Ontogenetic variation of color in preservative. Hatchlings and juveniles having dorsum of head and body regularly green, nuchal collar creamish-white spaced two to three dorsal scales.
Color in life. Dorsum of head dark brown gradually changing to olive green from midbody to posterior third of body; supralabials and gular region yellowish-cream; first two dorsal scale rows reddish-brown; belly and tail yellowish-cream ( Fig. 6B View FIGURE 6 ).
Hemipenial morphology (everted organ n= 4). Fully everted and maximally expanded hemipenes renders a slightly bilobed, non-calyculated, and non-capitate organs; hemipenis with smooth apical disk at surface of lobes; body covered by spinules, more concentrated on the marginal region of sulcus spermaticus; lobes show spinules on lateral region of organ; sulcus spermaticus bifurcates at the basal region of body, branches with centrifugal orientation extending toward central region of apical disks; basal region of sulcus spermaticus with an inflated portion ornamented with elongated spines; intrasulcal region with elongated spines arranged in rows extending to distal region of lobes; proximal region of body with bulges on either sides, ornamented by elongated spines; medial region of hemipenial body on the asulcate face ornamented with spinules homogeneously distributed; region around intrasulcal spines shows an area without ornamentation ( Fig. 3B View FIGURE 3 ).
Geographic distribution. Erythrolamprus oligolepis occurs widely on Brazilian Amazonia near Brazil / Peru frontier at Iquitos region. The species is also frequently registered in the southern Amazonia, and the only records from the left bank of the Amazon River are from northern Pará and Amapá states ( Fig. 8 View FIGURE 8 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Erythrolamprus oligolepis ( Boulenger 1905 )
| Ascenso, Alexandre C., Costa, João C. L. & Prudente, Ana L. C. 2019 |
Leimadophis oligolepis
| Cunha & Nascimento 1993 |
Liophis reginae semilineata
| Dixon 1983a |
Liophis oligolepis
| Boulenger 1905 |
Mus
| Linnaeus 1758 |