Nanotyrannus lancensis (Gilmore, 1946)
|
publication ID |
https://doi.org/10.1016/j.cretres.2015.12.016 |
|
DOI |
https://doi.org/10.5281/zenodo.4715039 |
|
persistent identifier |
https://treatment.plazi.org/id/03DC7E37-F05A-FFA3-FF49-FE18FBD9FA1D |
|
treatment provided by |
Jeremy |
|
scientific name |
Nanotyrannus lancensis |
| status |
|
The phylogenetic distribution of the lateral dentary groove was investigated to determine the polarity of the character, to interpret the implication of its losses, and to assess its potential as a diagnostic feature for re-classifying the position of “Jane” (BMR P2002.4.1) and other specimens labeled Nanotyrannus within Tyrannosauroidea. “Jane” is clearly a tyrannosaurid ( Brusatte et al., 2010), but its taxonomic affiliation has been highly disputed ( Erickson et al., 2006; Snively and Russell, 2007; Larson, 2008; Henderson and Harrison, 2008; Peterson et al., 2009; Larson, 2013a).
The debate over “Jane” is a smaller part of a larger debate about the validity of Nanotyrannus lancensis as a separate genus ( Bakker et al., 1988).
Three specimens have taken center stage in this debate: “ Jane ”, the holotype specimen of N. lancensis ( CMNH 7531 ), and the theropod described as one of the “Dueling Dinosaurs” ( BHI 6437 ; Larson, 2013b). The debate presently rests on three competing hypotheses, either that 1) Nanotyrannus stands as a valid taxon ( Currie, 2003a; Larson, 2013a); 2) these individuals represent the taxon Tyrannosaurus lancensis ( Currie et al., 2005); or 3) these individuals are juvenile Tyrannosaurus rex ( Carr, 1999; Brochu, 2003; Carr and Williamson, 2004; Holtz, 2004). A goal of this study is to attempt to clarify the relationship of N. lancensis with other tyrannosaurids using the dentary groove and other cranial characters ( Larson, 2013a).
2. Materials and methods
We investigated 92 theropod taxa for presence or absence of a groove on the lateral surface of the dentary ( Fig. 1 View Fig ). We also examined photographs of the crocodylomorph Shuvosaurus , the early dinosaurs Herrerasaurus and Staurikosaurus , and the sauropodomorphs Eoraptor, Leyesaurus, Pampadromaeus, and Panphagia for comparison. Sampled taxa and reviewed published records are delineated in the supplemental data View . We relied on descriptions of holotypes whenever possible, to avoid controversy as to taxonomic assignment. Some sampled specimens are the subject of debate (e.g. “Jane”) and the attribution of some unpublished specimens is controversial (e.g., assignment of specimens to Spinosaurus aegypticus). When such published descriptions did not include photographs of the specimen(s) or presence or absence of the groove was not unambiguously depicted in an illustration, we examined images of additional congeneric specimens, as available.
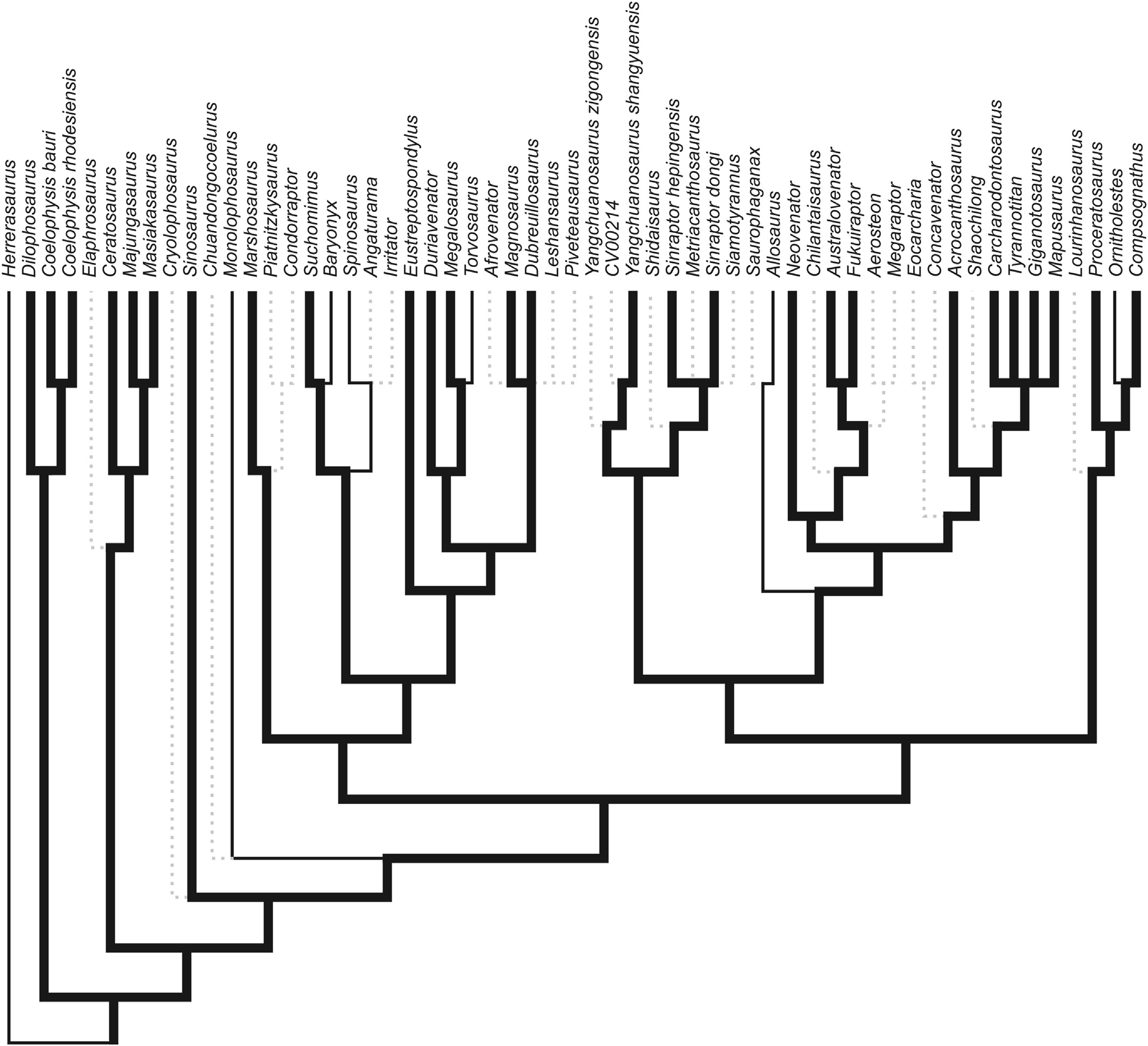
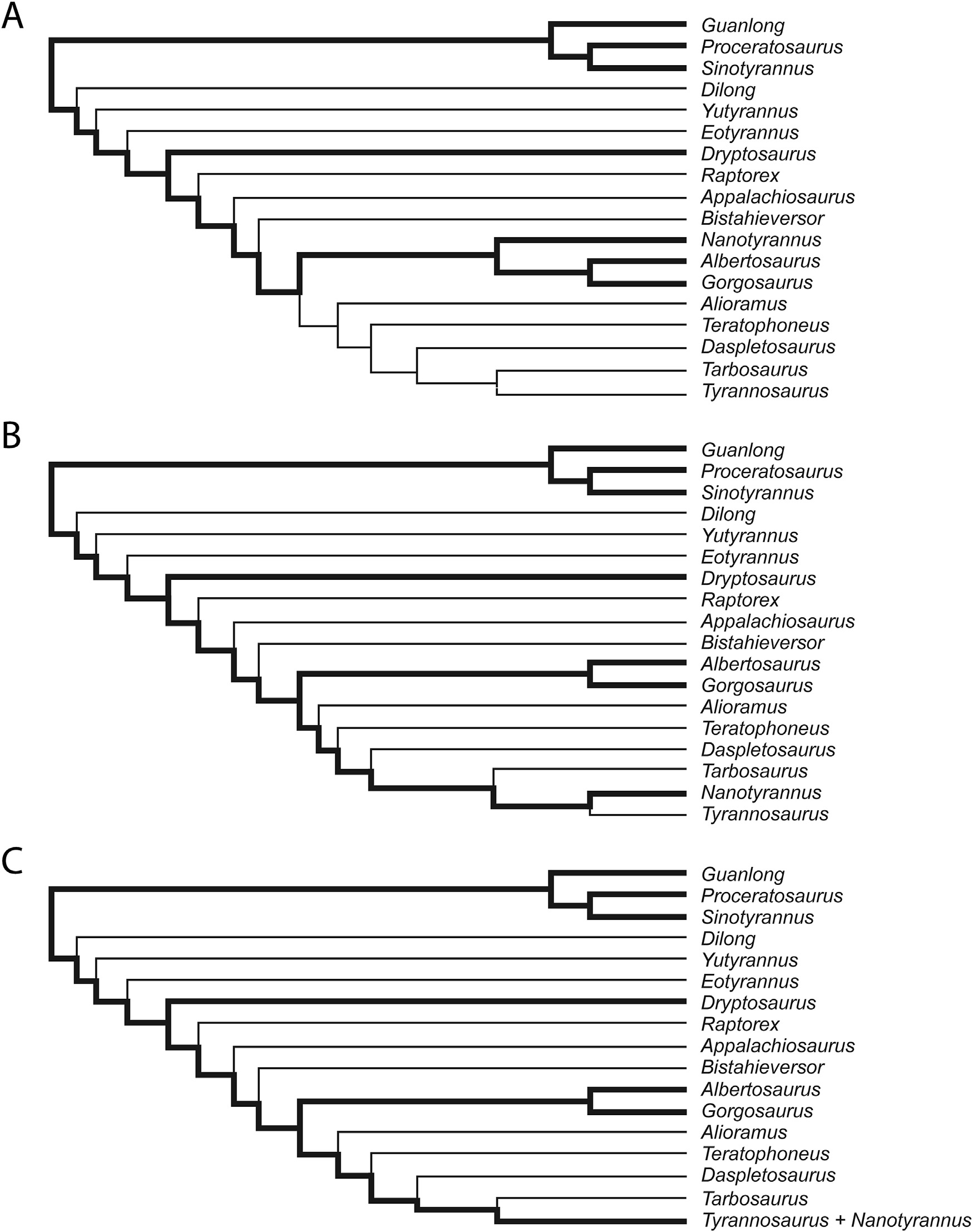
Phylogenetic analyses were conducted using TNT v1.1 ( Goloboff et al., 2008). We utilized the methodology and character matrix of Brusatte et al. (2010), and modified it only by adding Nanotyrannus to the character matrix and coding the dentary groove as present in Dryptosaurus, without modification of any other characters. The character matrix is included in the supplemental file View . We coded the character matrix for Nanotyrannus based on personal observations of skull of “Jane” at the Burpee Museum. Presence of the groove is character 176 of Brusatte et al. (2010) and character 124 of Carrano et al. (2012). We traced the most parsimonious occurrence of this character on the branches of these previously published cladograms to evaluate its use as a diagnostic character in theropods and to evaluate competing phylogenetic hypotheses ( Figs. 2 View Fig - 3 View Fig ). The most parsimonious result of this study is reported in Fig. 3A View Fig .
Institutional Abbreviations―LACM, Los Angeles County Museum, Los Angeles, CA; BHI, Black Hills Institute of Geological Research, Inc., Hill City, SD; BMR, Burpee Museum, Rockford, IL; BSP, Bayerische Staatsammlung für Paläontologie und historische Geologie, Munich, Germany; CMNH, Cleveland Museum of Natural History, Cleveland, OH; FMNH, Field Museum of Natural History, Chicago, IL; KUVP, University of Kansas Museum of Natural History and Biodiversity Institute, Lawrence, KS; NMMNH, New Mexico Museum of Natural History, Albuquerque, NM; TCM, The Children's Museum, Indianapolis, IN.
3. Results
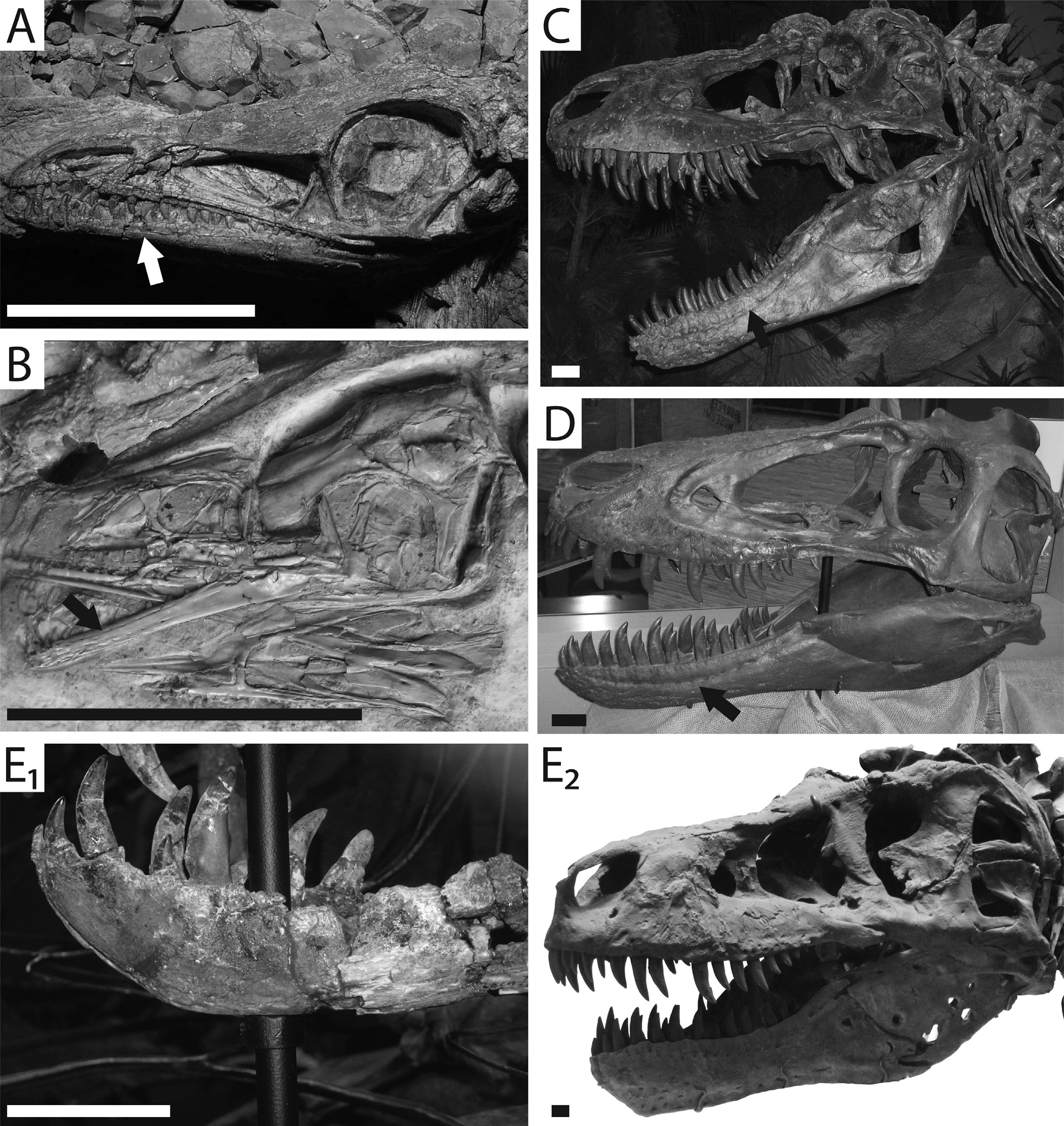
The dentary groove is a unique structure with multiple pores aligned within the depression and sub-equally spaced. The groove originates near the anterior portion of the bone from a position approximately underneath the 2nd to 4th dentary alveolus. It extends caudally along the lateral surface, terminating near the end of the tooth row ( Fig. 1 View Fig ; see also figure 1a of Sampson and Witmer, 2007). This groove does not extend beyond the dentary in examined theropods. This contrasts with the sauropodomorphs Eoraptor and Panphagia , wherein a groove extends onto the surface of the surangular and terminates in a large foramen. Multiple pores were noted along the length of the groove in theropod dinosaurs.
The dentary groove was present on 48 of 92 sampled theropod taxa (see supplemental data View ). No groove was observed in the crocodylomorph Shuvosaurus or the early dinosaur Herrerasaurus , strengthening the hypothesis that this feature is unique to theropod taxa. The groove was also not observed in Daemonosaurus or Tawa, the most primitive true theropods that we sampled. Allosaurus , Baryonyx , Monolophosaurus , Ornitholestes , Spinosaurus , and Torvosaurus were the only observed pre-Tyrannoraptoran taxa that reverted to the primitive state ( Fig. 2 View Fig ).
The single most parsimonious tree recovered by our phylogenetic analysis of tyrannosauridae is reported in Fig. 3A View Fig . The minimum tree length was found after 5 replicates, and the most parsimonious tree has a length of 561. Nanotyrannus was recovered as the sister taxon to the Albertosaurinae . We recovered the same topology as reported by Brusatte et al. (2010), except that the Proceratosauridae was recovered as a polytomy. The most parsimonious tree we produced resulted in a polytomy, regardless of whether Nanotyrannus was included or not. Alternative nonparsimonious tree topologies based on our result but reflecting the proposed relationships of Nanotyrannus among the Tyrannosauroidea sensu Currie (2003a) and Carr (1999) are presented for comparison in Fig. 3B and C View Fig respectively.
We investigated undisputed Tyrannosaurus rex material pertaining to the different life stages (i.e., juvenile, subadult, and adult of Carr and Williamson, 2004). The dentary of the smallest known juvenile of T. rex (LACM 28471) lacks the dentary groove. Although its dentary is fragmentary, the anterior portion is preserved, and examination failed to reveal the presence of a dentary groove. None of the T. rex specimens examined (LACM 28471, LACM 23845, LACM 150167, KUVP 155809, FMNH PR2081) possessed the groove.
4. Discussion
4.1. Discussion of presence of dentary groove
The dentary groove is present in 33 of 41 (80%) of the pre- Tyrannoraptoran theropods sampled here. We interpret only 6 reversals responsible for groove losses ( Fig. 2 View Fig ). The presence of this groove is therefore a character primitive for nearly all early theropods. Given the absence of the groove in the theropod clades which include Daemonosaurus and Tawa, the groove first evolved in the earliest common ancestor of the Neotheropoda. Within highly derived theropods, the dentary groove is present in some members of the Maniraptora. A similar foramina-bearing groove has been considered a synapomophy of Troodontidae ( Makovicky and Norell, 2004) . As such, it is present on all sampled troodontids, but limited to the posterior portion of the dentary. A groove was also observed in the dromaeosaurids Acheroraptor , Austroraptor and Buitreraptor . In Maniraptora, the groove appears to increase in height posteriorly (i.e., grows closer to the tooth row), creating a wedge-shaped appearance, rather than the linear shape of the groove in other theropod dinosaurs. In the troodontids, Acheroraptor , and Austroraptor , the groove originates beneath the middle of the tooth row and extends nearly to the posterior end of the dentary. We interpret this difference as truly morphological and do not consider the groove found in the Maniraptora to be homologous with the dentary groove of other theropods. Currie (1987) and Makovicky and Norell (2004) have interpreted a neurovascular function for this troodontid groove and its pores, an interpretation that we do not challenge.
Few tyrannosauroids retain this groove. “Jane” clearly has this groove ( Fig. 1D View Fig ) as does BHI 6437 . The condition of the Nanotyrannus holotype is unclear, as its jaws are occluded and the groove-bearing portion of the dentary obscured. The only other tyrannosauroids that possess this groove are Dryptosaurus aquilunguis and the Albertosaurinae : Gorgosaurus libratus and Albertosaurus sarcophagus.
4.2. Possible implications of beak formation and dentary groove absence
There are several dentary groove absences that occur simultaneously with the presence of a beak or edentulous jaws. The beaked ceratosaur Limusaurus, ornithomimids ( Nqwebasaurus and Shenzhousaurus), therizinosaurs (Erlikosaurus and Jiangchanosaurus), oviraptorosaurs ( Caudipteryx and Chirostenotes ) and all sampled birds lack the dentary groove. The jaw structure of Limusaurus has been completely modified into a beak and the dentary of Limusaurus even lacks any foramina on its lateral surface. Simply modifying the mandible into a beak, however, does not account for this absence of the groove. Only the anterior of the upper jaws were beak-like in Jiangchanosaurus, in which only the premaxillae were considered to have been covered by a rhamphotheca ( Pu et al., 2013). Jiangchanosaurus does not have a groove. The toothed birds Archaeopteryx, Hesperornis, Ichthyornis, and Parahesperornis also lack a dentary groove, despite being beakless or only having an incipient beak.
Adoption of herbivory is a reasonable hypothesis for the evolutionary loss of serrated teeth from the jaws in favor of edentulous jaws or beaks. One explanation is that that the dentary groove played a role in predatory behavior and that the groove was lost in these theropods because carnivory was abandoned. Ornithomimids ( Kobayashi et al., 1999; Norell et al., 2001) and therizinosaurs ( Zanno et al., 2009) are both postulated to have been herbivorous. Oviraptorosaurs may have been predominately herbivorous ( Ji et al., 1998; Xu et al., 2002), although they may have taken prey or scavenged under rare circumstances ( Norell et al., 1994). Analysis of jaw mechanics in theropod dinosaurs clearly demonstrates the dissimilarity of oviraptosaurs and predatory theropods ( Brusatte et al., 2012b). The lack of the dentary groove is taken as further evidence that they may not have been active predators. The possibility that the common ancestor of Ornithomimosaurs, Oviraptorosaurs, and Therizinosaurs also lacked a dentary groove cannot be overlooked, especially given the absence of a groove in most other maniraptorans. While the presence of a beak does not necessarily indicate herbivorous lifestyle―as squid, placoderm fish and birds of prey all utilize beaks in predatory behavior―why a well-adapted predator like a theropod would evolve a beak to bolster a predatory lifestyle is unclear, and an interpretation of herbivory in this context is more parsimonious.
4.3. Absence of groove in tyrannosauroids
There are numerous tyrannosauroids that lacked the dentary groove. Assuming maximum parsimony for the distribution of the dentary groove, we must interpret several of these as independent losses. One potential explanation for the absence of the groove in certain taxa might be that the nerves and blood vessels accommodated by the groove occurred superficial to the dentary rather than along its surface. Another potential explanation for the loss of the groove in various tyrannosaurids is to enhance jaw strength. The groove could potentially serve as a point of weakness: forcemodeling experiments on the mandible of Erlikosaurus demonstrate higher stress levels along the line of mandibular foramina during simulated bites ( Lautenschalger et al., 2013). The groove was likely eliminated as the cortical bone of the dentary was thickened to accommodate strong bite forces ( Therrien et al., 2005). Loss of the groove would therefore be advantageous from a mechanical perspective for the largest tyrannosaurids. Snively et al. (2006) hypothesized that tyrannosaurines had stronger bite forces compared to albertosaurines and carnosaurs, due to vaulting of the skull, enhanced fusion of the cranial bones and skull kinematics. Tyrannosaurines likely consumed greater amounts of bone relative to smaller theropods ( Fiorillo, 1991; Erickson and Olson, 1996; Snively et al., 2006). That makes sense, given that (1) observation of modern ecosystems demonstrates that predators with stronger bite forces tend to consume more portions of the prey ( Van Valkenburgh, 1996) and (2) the presence of prey items (e.g., ceratopsians) in the Cretaceous which possess bony frills. Large tyrannosaurids used a “puncture and pull” feeding strategy ( Bakker, 1986; Molnar and Farlow, 1990; Erickson and Olson, 1996) which required that the teeth penetrate bone, in order to effectively anchor the jaws into the prey. This feeding behavior has been interpreted from bite marks on ceratopsians ( Erickson and Olson, 1996). Other theropods (e.g, Allosaurus , Giganotosaurus ) may have instead relied on cranial kinesis and thin, slicing teeth to wound their prey ( Paul, 1988; Rayfield et al., 2001; Therrien et al. 2005), instead of ripping large portions (e.g., limbs) from the body of the prey. Since the groove conducted a nerve along the surface of the dentary, a powerful bite may also have been intrinsically painful. The nerve may have been reduced and the groove therefore eliminated, as more powerful biting force evolved in tyrannosaurids. A biomechanical explanation for the loss of the groove is appealing, because large carcharodontosaurids were stratigraphically contemporaneous with tyrannosaurines and, in some cases, were larger than tyrannosaurines. Thus increase in size alone is not sufficient to explain the loss of the groove.
The variable distribution of the groove among tyrannosaurids may explain the seemingly incompatible cohabitation ( Farlow and Pianka, 2003) of Daspletosaurus (in which a groove is absent) with Gorgosaurus and Albertosaurus (in which the groove is present). Daspletosaurus may have been adapted to eating prey that required powerful bites to subdue or consume, whereas the albertosaurines were more lightly built predators that perhaps preferred less robust prey. This is in keeping with Russell's (1970) hypothesis that albertosaurines preyed on hadrosaurs, whereas Daspletosaurus specialized on ceratopsians. Tyrannosaurines are known to have taken hadrosaurs as prey items ( Varricchio, 2001; DePalma et al., 2013). Rather than invalidate our hypothesis, this merely is evidence that tyrannosaurines were large, powerfully built predators that were capable of taking any suitable prey items in their environment. This explanation is consistent with the mechanical hypothesis we pose here.
A potential flaw in this hypothesis is the absence of the groove in many smaller tyrannosauroid genera, such as Alioramus, Dilong, and Eotyrannus. While an examination of the bite force of these taxa is beyond the scope of the present study, it may be the case that these smaller tyrannosauroids were also exerting relatively high bite forces on their prey or otherwise needed to strengthen their jaws for successful food acquisition.
4.4. Interpreting the identity of “ Jane ” and the validity of Nanotyrannus
The mosaic distribution of the dentary groove within Tyrannosauroidea allows an opportunity to use the occurrence of this character to determine the relationship of the embattled Nanotyrannus to other tyrannosaurs. Tyrannosaurus rex lacks this groove, whereas it is a distinct character in “Jane” and the other Nanotyrannus lancensis specimens in which the dentary is visible. The occurrence of the dentary groove in N. lancensis , but not in T. rex , is itself independent evidence of separation of these two taxa. The presence of the groove stands with more than 30 other skeletal characters as evidence to separate Nanotyrannus from Tyrannosaurus ( Larson, 2013a) .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Albertosaurinae |
|
Genus |