Harpactea cressa Brignoli, 1984
|
publication ID |
https://doi.org/ 10.5281/zenodo.2645543 |
|
persistent identifier |
https://treatment.plazi.org/id/03DC87A1-FFA5-FFC7-FEA0-F90782DCC1CD |
|
treatment provided by |
Plazi |
|
scientific name |
Harpactea cressa Brignoli, 1984 |
| status |
|
Harpactea cressa Brignoli, 1984 View in CoL
( Figs 21–29 View FIGURES 21–24 View FIGURES 25–29 , 41 View FIGURE 40–42 )
Harpactea cressa Brignoli 1984: 285 View in CoL , figs. 4–5 (male). Holotype male from Mt. Aloitha above Mesa Lathitakion, 1000 m, Lasithi , Crete; in soil under Quercus coccifera View in CoL ; 6.iv.1978; coll. B. Hauser; stored at MHNG (Kar79/24); examined.
Material examined
Additional material examined. Chania: Site 1 (a 3 ♂♂; b 2 ♂♂); Site 2 (a 1 ♂); Site 3 (a 1 ♂; b 4 ♂♂ 3 ♀♀); Site 4 (a 1 ♂; b 1 ♂); Site 5 (a 3 ♂♂ 1 ♀); Site 6 (a 2 ♂♂ 1 ♀); Site 7 (a 1 ♂); Site 8 (a 1 ♂ 1 ♀); Site 9 (a 2 ♀♀; b 2 ♂♂; c 2 ♂♂ 1 ♀); Site 10 (a 1 ♂); Site 11 (a 1 ♂; b 1 ♂); Site 12 (b 4 ♂♂; c 1 ♂; g 3 ♂♂; h 1 ♂); Site 14 (c 2 ♂♂); Site 15 (a 3 ♂♂ 3 ♀♀; b 7 ♂♂ 2 ♀♀; c 12 ♂♂ 2 ♀♀; e 2 ♂♂); Site 16 (b 2 ♂♂; d 1 ♂); Site 17 (b 1 ♂ 2 ♀♀); Site 19 (a 2 ♂♂; d 1 ♀; e 1 ♂; f 11 ♂♂ 1 ♀); Site 20 (a 3 ♂♂; c 1 ♂; d 1 ♂; e 2 ♀♀); Rethymno: Site 21 (a 1 ♂ 1 ♀; b 1 ♂ 2 ♀♀); Site 22 (a 8 ♂♂ 4 ♀♀ [ MHNG]; b 9 ♂♂; d 1 ♂ 1 ♀; e 2 ♂♂ 2 ♀♀); Site 23 (a 6 ♂♂) ; Site 25 (a 5 ♂♂); Site 26 (a 4 ♂♂); Site 28 (a 5 ♂♂); Site 29 (b 1 ♂ 1 ♀; c 4 ♂♂ 1 ♀); Site 31 (a 4 ♂♂ 3 ♀♀); Site 32 (a 26 ♂♂ 1 ♀); Site 33 (a 1 ♂); Irakleio: Site 36 (a 2 ♂♂); Site 37 (a 1 ♂); Site 38 (b 6 ♂♂); Lasithi: Site 41 (a 1 ♂); Site 44 (a 1 ♂ 2 ♀♀; b 1 ♂); Site 47 (a 4 ♂♂); Site 49 (a 3 ♂♂); Site 50 (a 2 ♂♂).
Diagnosis
Can be distinguished from all other Cretan Harpacteinae by its vulva with tubelike posterior diverticulum, distinctly curved upwards and long, thin spermatheca, very similar but thinner than that of H. coccifera . Male bulb with thick, transverse, proximally bent embolus similar to H. catholica but distinguished from this species by lamellalike, bifurcated conductor, and accessory apophysis that is flat, rectanglelike and bent backwards. Differs from similar North Anatolian H. osellai Brignoli, 1978 , by conductor and accessory apophysis shape (hooklike conductor; short, fingerlike AA in H. osellai ) and embolus trajectory (upwards in H. osellai ).
Description
MALE. Small to medium sized ( Table 7). Carapace with smooth surface, reddish brown and hairs more densely distributed at cephalic part. Apparent longitudinal fovea. Carapace lateral margins clearly angled at its widest point, in dorsal view. PME smaller than the rest of eyes, separated from PLE by 0.5 PLE’s diameter. Distance between AME equal to their diameter. Labium length two times its width, with hairy whitish upper part. Sternum yellow with hairs on the periphery.
Chelicerae with setiferous granulations along their whole frontal surface. Retromargin with two small teeth, a tiny one at the base of the groove and another one larger on its middle part ( Fig. 22 View FIGURES 21–24 ). Promargin with two teeth of equal size, close to each other. Proximalmost tooth of the retromargin located in the interspace of the two at the promargin or opposite to the distalmost tooth of the promargin.
Abdomen yellowish. Light hairs equally distributed along the whole abdomen. Femur I–II reddish brown, all other segments and legs III–IV yellow. Leg spination and measurements (see Tables 8–9 View TABLE 8 View TABLE 9 ).
Male bulb ( Figs 21 View FIGURES 21–24 , 25–29 View FIGURES 25–29 ): Tibia straight, of equal size as tarsus. Tarsus triangular in lateral view, forming a small conical tip on the base of the tegulum. Tegulum relatively enlarged at its apical part. Embolar division large compared with tegulum size, with broad embolar base. Embolus transverse, thick. Conductor bifurcated with a lamellalike large plate and a very slender, sharp projection. Rectangular accessory apophysis, slightly bending on its apical part.
Female. All characters as described for male. Vulva ( Figs 23–24 View FIGURES 21–24 ): Long, tubelike posterior diverticulum abruptly turning on its mid to distal part. Long and slender, sclerotized spermatheca with rather broad dorsal base and pointed tip, encircled in a transparent membrane. In profile, the upper part of this sclerotization is enlarged, very similar to H. coccifera . Small anterior arc. Transversal bar indistinguishable.
Intraspecific variation
H. cressa on Crete are small ( Table 7). Some of the individuals from Gavdos island fall in the same size range, while others are much larger (AL: 2.4–3.1, CL: 3, CWmax: 2.2).
Ecology
Found from coastal phrygana up to 1450m altitude, often in Quercus , Cupressus and Pinus forests of Crete. Active from early spring to May and in mid fall (October November). Possible peak of activity in MarchApril.
Distribution Widespread on Crete and on Gavdos. On the latter, it is the only representative of the genus.
Genus Stalagtia Kratochvil, 1970
Stalagtia thaleriana n. sp.
( Figs 30–39 View FIGURES 30–34 View FIGURES 35–39 , 42 View FIGURE 40–42 )
Material examined
Types. 1 ♂ holotype; 15 ♂♂, 20 ♀♀ paratypes [NHMC81.2.1026.1]; 1 ♂, 1 ♀ paratypes [ MHNG]; 1 ♂, 1 ♀ paratypes [ CRBA]. Type and paratype locality: Ano Meros, Rethymno , Crete (pitfalls sampling period: 5.x.99 to 26.i.00)
Additional material examined. Chania: Site 10 (a 3 ♂♂ 1 ♀); Site 11 (a 1 ♂); Site 12 (a 7 ♂♂ 5 ♀♀; d 7 ♀♀; f 1 ♂ 1 ♀; g 8 ♂♂ 6 ♀♀; h 11 ♂♂ 3 ♀♀); Site 13 (b 1 ♂; c 1 ♂); Site 14 (a 4 ♂♂ 6 ♀♀; b 15 ♂♂; c 29 ♂♂ 4 ♀♀; d 7 ♂♂ 2 ♀♀; e 1 ♂; f 3 ♂♂ 11 ♀♀; g 6 ♂♂ 1 ♀); Site 15 (a 15 ♂♂ 4 ♀♀; b 7 ♂♂ 4 ♀♀; e 1 ♂ 1 ♀); Site 16 (a 26 ♂♂ 4 ♀♀; b 4 ♂♂; d 1 ♂ 1 ♀; e 5 ♂♂ 3 ♀♀); Site 17 (a 4 ♂♂ 9 ♀♀; b 31 ♂♂ 6 ♀♀); Site 18 (a 5 ♂♂ 5 ♀♀; b 4 ♂♂ 3 ♀♀); Site 19 (a 1 ♂ 9 ♀♀; b 6 ♀♀; c 2 ♀♀; d 5 ♀♀; e 5 ♂♂ 2 ♀♀; f 13 ♂♂ 2 ♀♀); Site 20 (a 23 ♂♂ 3 ♀♀; b 3 ♂♂); Rethymno: Site 21 (a 2 ♂♂ 3 ♀♀; b 16 ♂♂ 7 ♀♀); Site 22 (a 36 ♂♂ 5 ♀♀; b 3 ♂♂; c 1 ♀; e 6 ♀♀); Site 25 (a 14 ♂♂); Site 26 (a 2 ♂♂ 4 ♀♀); Site 28 (a 40 ♂♂ 2 ♀♀); Site 29 (a 1 ♂ 3 ♀♀; b 11 ♂♂ 8 ♀♀; c 3 ♂♂); Site 30 (a 3 ♂♂ 1 ♀; b 8 ♂♂ 2 ♀♀; c 16 ♂♂ 1 ♀); Site 31 (a 47 ♂♂ 13 ♀♀; b 14 ♂♂ 15 ♀♀); Site 32 (a 28 ♂♂ 2 ♀♀); Site 33 (a 11 ♂♂ 2 ♀♀); Site 34 (a 3 ♂♂ 2 ♀♀; b 4 ♂♂ 4 ♀♀); Site 35 (a 1 ♀); Irakleio: Site 36 (a 7 ♂♂ 18 ♀♀); Site 37 (a 8 ♀♀; b; c 5 ♂♂ 18 ♀♀); Site 38 (a 7 ♂♂ 1 ♀; b 1 ♂); Site 39 (a 1 ♂ 1 ♀); Site 40 (a 27 ♂♂ 6 ♀♀; b 11 ♂♂ 17 ♀♀); Lasithi: Site 41 (b 27 ♂♂ 1 ♀); Site 42 (a 1 ♂ 1 ♀; b 19 ♂♂); Site 43 (a 2 ♀♀; b 2 ♂♂ 3 ♀♀; c 5 ♂♂ 3 ♀♀); Site 44 (a 12 ♂♂ 4 ♀♀; b 1 ♂ 6 ♀♀); Site 45 (a 2 ♂♂ 2 ♀♀); Site 46 (a 2 ♀♀); Site 47 (a 2 ♂♂ 2 ♀♀; b 2 ♂♂ 1 ♀); Site 48 (a 4 ♀♀).
Comparative material examined. Stalagtia kratochvili Brignoli, 1976 : Ipeiros: close to Filiatis, 170 m, pine forest (1 ♂ Holotype, MHNG: Ep73/86); close to Megalo Peristeri, 620 m, under Quercus (3 ♀♀ paratypes, MHNG: Ep73/34); 3 km after Karies, towards E lati, 740 m, under Quercus (2 ♀♀ paratypes, MHNG: Ep73/17); 11Km SE of Konitsa, gorge in Voithomatis, 450 m, under Quercus and Castanea (1 ♀ paratype, MHNG: Ep73/ 50); Sterea Ellada: Voiotia: road to Arachova, Eptalofos, 1280m (1 ♀, MHNG: The76/ 10).
Etymology The new species is dedicated to the late Austrian arachnologist Konrad Thaler, who made a great contribution to knowledge of Cretan spiders.
Diagnosis
Differs from any other Harpacteinae in Crete by the very simple male bulb (accessory apophysis absent and conductor reduced to small toothlike apophysis at the base of the embolus), and a vulva with a short, globular posterior diverticulum and a small, distal keellike projection in the spermatheca. Differs from other Stalagtia species by slender and long embolus, which is shorter than the tegulum slightly curved forward and distally markedly bent.
Description
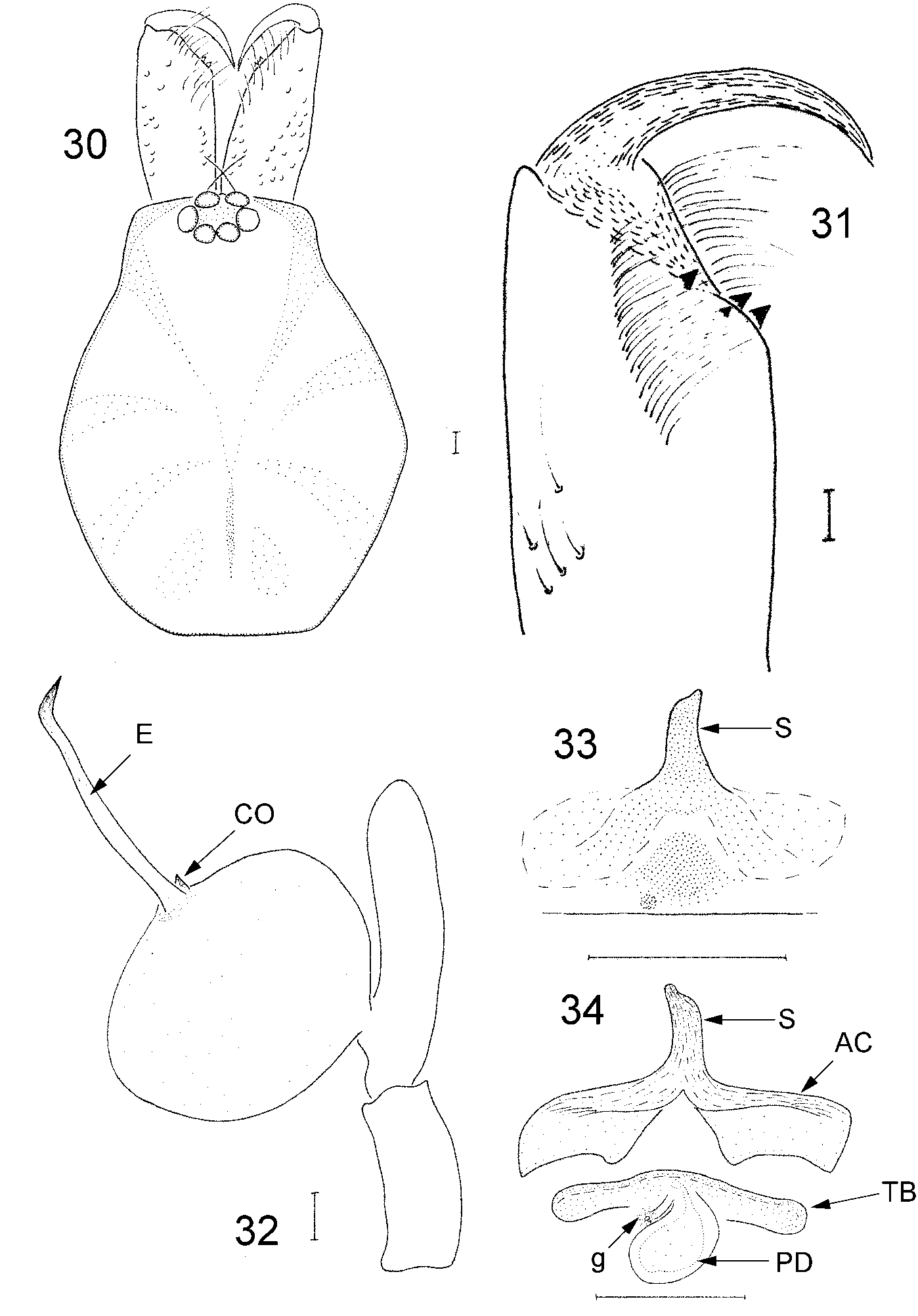
Male holotype ( Fig. 30 View FIGURES 30–34 ). Carapace orange to light brown, smooth, scarcely granulated at anterior part. Cephalic part narrower, clearly differentiated from the thoracic part ( Table 10 View TABLE 10 ). Fovea apparent, covering almost half of the thoracic part. Eyes very close to each other, the space between AME less than half their diameter.
Chelicerae with setiferous granulations on dorsal part that become more dense on lateral sides. Basal part of chelicerae hairless, groove of chelicerae covered with hairs arranged in two lines. Retromargin with one tooth on the base of the groove and another one of equal size on its middle part ( Fig. 31 View FIGURES 30–34 ). Promargin with two teeth of equal size, larger than those at the retromargin. Proximalmost tooth of the retromargin located in the interspace of the two at the promargin or opposite to the distalmost tooth of the promargin, very similar to H. cressa (except for a difference in relative size of teeth). Body, cheliceral and ocular measurements are shown in Table 10 View TABLE 10 . Legs with same color as carapace. Leg spination and measurements are shown in Tables 11 View TABLE 11 and 12 View TABLE 12 , respectively. Relative leg length of male type and female paratype: IV>I>II>III.
Male bulb ( Figs 32 View FIGURES 30–34 , 35–39 View FIGURES 35–39 ): Tibia short, almost half of the tarsus length, slightly recurved. Simple palpal organ with globular bulb and a long, slender, spiniform embolus, ending in a curved tip. The base of the embolus holds a thornlike projection, possibly homologous to a conductor.
Female. All characters as described for male. Vulva ( Figs 33–34 View FIGURES 30–34 ): Small and sclerotized spermatheca, visible only by dissection of the genital organ, projected from a relatively large, sclerotized, inverted Yshaped anterior arc. Anterior arc with curved ventral edge and paddleshaped dorsal base. Posterior diverticulum small, oval shaped, with a glandular formation at its basal part.
Ecology
Found in coastal phrygana up to 1950 m. Also widespread in Quercus , Cupressus and Pinus forests of Crete. Active all year round except for the very dry summer months (although few specimens were caught in midJuly). Highest activity MarchApril and OctoberNovember.
Distribution Widespread on the western and central part of Crete; absent from the easternmost sites.
Key for identification
1. Distance between AME smaller than 0.5 AME diameter ............................................. 2 Distance between AME equal or larger than 0.5 AME diameter.................................. 3 2. Tibia of pedipalp longer than tarsus. Patellae spineless. All cheliceral teeth of equal size, those at promargin not close together .................................... Harpactea coccifera View in CoL Tibia of pedipalp shorter than tarsus. Patella III with 1–2 dorsal spine. Proximal most tooth of retromargin tiny ................................................................ Stalagtia thaleriana View in CoL 3. Anterior tibiae and metatarsi spineless. Patella III with one dorsal spine. Patella IV spineless. Proximal most tooth of retromargin tiny ............................ Harpactea cressa View in CoL Anterior tibiae and metatarsi with several ventral spines. Patella III with one ventral spine. Patella IV with one ventral and one dorsal spine ................. Harpactea catholica View in CoL
Discussion
All Harpacteinae presented in this paper share the same habitats and periods of activity. H. catholica is mostly restricted to woody environments and caves, while the other species also inhabit the most common habitats of Crete, i.e. those less densely vegetated and lacking leaf litter shrublands. Among Harpacteinae, H. cressa and S. thaleriana n. sp. are the dominant species, the former being the only representative of the subfamily on the islands of Gavdos and Gavdopoula. Given this observation and also the finding that these two species showed high presence in the drier months, we propose that these species are the most resistant to aridity, although they are mostly active in early spring and in midautumn, like the other species studied. In all cases, males and females occur more or less at the same time period, the males being active during a longer period than females. However, this observation is probably an artifact of the collection method (mostly pitfalls), males are more frequently collected because they are actively searching for mates. In Stalagtia thaleriana n.sp., for which we have the largest collections, male peaks preceded those of females in some localities, but this is not a general rule. Gaps in the distribution of the two most common species ( H. cressa and S. thaleriana n. sp.) on Crete are probably the result of restricted sampling in some areas, such as the lowlands of western and central Crete. Conversely, the absence of S. thaleriana n. sp. and H. catholica in eastern Crete is supported by the thorough examination of the area, which did not yield a single capture of either. The distributional pattern of S. thaleriana n. sp. closely matches that reported for other spider species on Crete, namely the gnaphosids Trachyzelotes lyonneti (Audouin, 1826) , Drassyllus pumiloides Chatzaki, 2003 , Drassyllus praeficus (L. Koch, 1866) , Callilepis cretica (Roewer, 1928) , Haplodrassus dalmatensis (L. Koch, 1866) ( Chatzaki et al. 2002a, 2002b, 2003). This pattern may be related to ecological (extreme aridity during a long dry season, Pennas 1977) and historical factors (the eastern part of Crete after Ierapetra was isolated from the rest of the island until very recently, Dermitzakis 1981). There is no evidence of size segregation among the species studied. Size ranges mostly overlapped, and all species fall in small to medium size categories. Overall, H. cressa is the smallest representative on Crete and H. catholica the largest.
The three species of Harpactea found on Crete can be assigned to two of the four species groups proposed by DeelemanReinhold (1993). H. coccifera belongs to the “ hombergi ” group and closely resembles H. hombergi (Scopoli, 1763) , the type species of the genus, in male bulb morphology. This species group is distributed across Europe and North Africa. Species showing closer geographical and morphological affinities with H. coccifera include H. villehardouini Brignoli 1979 from Peloponnisos and H. nausicaae Brignoli, 1976 from Ipeiros, Kerkyra (Corfu) and Kefalonia ( Brignoli 1984). Male bulb characters and leg spination indicate that H. cressa is a member of the “ rubicunda ” group (contra Brignoli 1984). However, the narrow, tubular posterior diverticulum of the vulva does not conform to the general pattern of the group, which is characterized by wide posterior diverticula. The male bulb pattern and the shape of the spermatheca of this species closely resemble those of H. osellai Brignoli, 1978 from the Pontic Mountains (Amasya) in Turkey. These two speciesformerly included in Brignoli’s group “ babori ”differ from other species of the “ rubicunda ” group in the shape of the tegulum, which is clearly longer than wide in these two species and globular in the remaining ones.
The morphological affinities of H. catholica are a matter of debate. This species was originally classified a member of the genus Minotauria ( Brignoli, 1984) on the basis mostly of the leg spination of a single female specimen found in Katholiko cave, near Chania. It was later transferred to the Harpactea , group “ rubicunda ”, after study of newly collected male material and cheliceral dentition ( DeelemanReinhold 1993). According to DeelemanReinhold (1993), Harpactea are distinguished from the microphtalm (or eyeless) genera Folkia and Stalagtia by the absence of spines in the anterior tibiae and metatarsi, which are present in H. catholica . DeelemanReinhold (1993) explained this unusual character for Harpactea as an adaptation to the cave environment. The remarkable presence of spines on femora, tibiae and metatarsi of anterior legs, sometimes in high densities, has been reported in other cavedwelling Dysderidae such as the genus Stalita Schiödte, 1847 , and some troglobitic species of the genus Dysdera ( Arnedo & Ribera 1999) . However, this explanation seems questionable in the case of H. catholica since this species is also found in open ground. Curiously, the same author used the presence of spines in anterior legs as a character to restrict the delimitation of the genus Stalagtia ( DeelemanReinhold 1993) . In its present definition, the Harpacteinae genus Stalagtia is restricted to cavedwelling species with ventral spines on the anterior tibiae and metatarsi, spineless PaIII and CoIV, ballshaped male bulb with long, transverse embolus and small or absent conductor, and a small, inverted Y or Vshaped vulva with a wide posterior diverticulum ( DeelemanReinhold 1993). Only the Southern Dalmatian species, S. hercegovinensis (Nosek, 1905) and S. monospina (Absolon & Kratochvil, 1933) fit this narrow definition. The species H. argus ( Brignoli, 1976) was originally described as Stalagtia and was subsequently claimed to belong to the Harpactea group “ corticalis ” ( DeelemanReinhold 1993), while S. kratochvili Brignoli, 1976 was excluded from the genus Stalagtia and proposed to belong somewhere between the Harpactea species group “ rubicunda ” and the genus Dasumia , but without an explicit genus assignation ( DeelemanReinhold 1993). In our opinion, S. thaleriana n. sp. constitutes a clear link between the Greek species formerly considered Stalagtia (i.e. S. kratochvili and H. argus ), and the species currently included in the genus, and reveals that the current definition of Stalagtia is too restrictive. All these species share the same male bulb pattern: an embolus as long or longer than the tegulum, curved on its proximal side and projected forward (in S. thaleriana n. sp. the curve is slightly attenuated, while in the other species the embolus is almost transverse; in both S. thaleriana n. sp. and S. hercegovinensis the tip of the embolus is bent, although in different directions); the conductor is absent ( H. argus ), hardly visible ( S. thaleriana n. sp., S. hercegovinensis ) or small but clearly recognizable ( S. kratochvili ). The similarity of the vulvas of these species has been previously overlooked. An inverted Y or Vshaped vulva has been proposed as a diagnostic character of Stalagtia ( DeelemanReinhold 1993) . However, Brignoli’s drawings of S. kratochvil and H. arguta show a very similar shape. The pattern is once again repeated in S. thaleriana n. sp., although in this case the short arms of the inverted Y arc are slightly more open. Moreover, S. hercegovinensis , S. thaleriana n. sp., S. kratochvili and H. arguta (Simon, 1907) show a very similar posterior diverticulum: rounded, short (shorter than or as long as the spermatheca) and wrinkled. Major differences between these species are restricted to leg spination: ventral spines on anterior tibiae and metatarsi and spineless PaIII, CoIV in S. hercegovinensis and S. monospina and the opposite state in H. argus , S. kratochvili and S. thaleriana n. sp. H. catholica , a species that does not show any clear morphological affinity with Stalagtia species, other than the subfamily characters, also shows spines in the ventral side of the anterior tibiae and metatarsi. This observation alone is probably sufficient to reject the use of a particular spination pattern as an indication of generic status. Therefore, we formally propose transferring H. argus back to Stalagtia and omitting spination pattern as a diagnostic character of this genus. In fact, the genus Stalagtia is probably much more diverse than previously considered. In addition to several new species recently collected in Turkey and the Middle East (M. Ṙ ezáč, pers. comm.), several species currently included in Harpactea , more specifically species in Brignoli’s group “ abantia ”, should probably be transferred to Stalagtia .
The difficulties encountered regarding definition and species ascription are not exclusive of Stalagtia and can also be found in the remaining genera of Harpacteinae. Several species of Harpactea would better fit the genus Dasumia ( Alicata 1966a) . Moreover, some species of the “rubicunda” group maybe closer to Dasumia than to other Harpactea ( DeelemanReinhold 1993) . One of the main characters separating Minotauria from Harpactea is the position of cheliceral teeth. In Minotauria , the proximalmost tooth on the retromargin is situated opposite or distally to the distalmost tooth on the promargin, while in Harpactea it is found opposite the interspace of the teeth on the promargin. However, in both S. thaleriana n. sp. and H. cressa , the arrangement of cheliceral teeth can roughly be considered as belonging to the “classical” Harpactea Stalagtia group. In these cases, the relative position of the proximalmost tooth of the promargin to those of the retromargin is a matter of angle when observing chelicerae, therefore we propose that cheliceral teeth arrangement is not a robust diagnostic character. Finally, several characters of Folkia are quite similar to Stalagtia , for example a simple male bulb with conductor (well developed in Folkia ) but lacking accessory apophysis or the small globeshaped posterior diverticulum of the vulva.
A complete redefinition of the distinct genera and species groups included in the subfamily Harpacteinae is beyond the scope of this paper. However, here we show that Harpacteinae can be currently summarized as a set of narrowly defined genera, in some cases ecologically distinct, and a ragbag of species that are collectively referred to as Harpactea , because they cannot clearly be included in any other Harpacteinae genus. As a concluding remark, we wish to stress the importance of producing a working phylogenetic hypothesis of the Harpacteinae representatives in order to circumvent the current limitations of the taxonomy of this diverse spider group.
TABLE 10. Stalagtia thaleriana n. sp. Body measurements taken from the male holotype and one of the female paratypes. Parentheses show corresponding range of the main measurements of the body (8 males, 6 females, in mm).
| CL | CWmax | CWmin | AL | ChL | ChG | ChF | AMEd | PLEd | PMEd | |
|---|---|---|---|---|---|---|---|---|---|---|
| males | 2.1 (1.89–2.41) | 1.79 (1.47–1.89) | 0.95 (0.63–0.94) | 3.05 (2.31–3.0) | 0.92 | 0.31 | 0.51 | 0.14 | 0.12 | 0.12 |
| females | 2.42 (2.10–2.73) | 1.79 (1.58–2.10) | 0.95 (0.84–1.05) | 3.15 (2.52–5.04) | 1.02 | 0.36 | 0.46 | 0.14 | 0.12 | 0.12 |
| MHNG |
Museum d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Harpactea cressa Brignoli, 1984
| Chatzaki, Maria & Arnedo, Miquel A. 2006 |
Harpactea cressa
| Brignoli, P. M. 1984: 285 |